
Principles of Instrumental Analysis
7th Edition
ISBN: 9781305577213
Author: Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
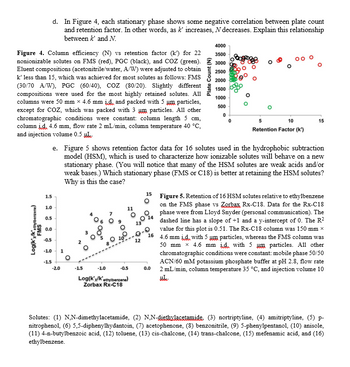
Transcribed Image Text:d. In Figure 4, each stationary phase shows some negative correlation between plate count
and retention factor. In other words, as k' increases, N decreases. Explain this relationship
between k' and N.
Plate Count (N)
4000
3500
2500
2000
1500
1000
Figure 4. Column efficiency (N) vs retention factor (k') for 22
nonionizable solutes on FMS (red), PGC (black), and COZ (green). 3000
Eluent compositions (acetonitrile/water, A/W) were adjusted to obtain
k' less than 15, which was achieved for most solutes as follows: FMS
(30/70 A/W), PGC (60/40), COZ (80/20). Slightly different
compositions were used for the most highly retained solutes. All
columns were 50 mm × 4.6 mm id and packed with 5 um particles,
except for COZ, which was packed with 3 um particles. All other
chromatographic conditions were constant: column length 5 cm,
column j.§. 4.6 mm, flow rate 2 mL/min, column temperature 40 °C,
and injection volume 0.5 μL
Log(k'x/K'ethylbenzene)
FMS
1.5
1.0
0.5
0.0
ཐྭ ཋ ཤྩ བྷྲ ;
500
0
5
10
Retention Factor (k')
15
e. Figure 5 shows retention factor data for 16 solutes used in the hydrophobic subtraction
model (HSM), which is used to characterize how ionizable solutes will behave on a new
stationary phase. (You will notice that many of the HSM solutes are weak acids and/or
weak bases.) Which stationary phase (FMS or C18) is better at retaining the HSM solutes?
Why is this the case?
12
15 Figure 5. Retention of 16 HSM solutes relative to ethylbenzene
-0.5
-1.0
1
-1.5
-2.0
-1.5
-1.0
-0.5
0.0
Log(k'x/k'ethylbenzene)
Zorbax Rx-C18
14
16
on the FMS phase vs Zorbax Rx-C18. Data for the Rx-C18
phase were from Lloyd Snyder (personal communication). The
dashed line has a slope of +1 and a y-intercept of 0. The R²
value for this plot is 0.51. The Rx-C18 column was 150 mm ×
4.6 mm id with 5 um particles, whereas the FMS column was
50 mm × 4.6 mm id with 5 um particles. All other
chromatographic conditions were constant: mobile phase 50/50
ACN/60 mM potassium phosphate buffer at pH 2.8, flow rate
2 mL/min, column temperature 35 °C, and injection volume 10
ut
Solutes: (1) N,N-dimethylacetamide, (2) N,N-diethylacetamide. (3) nortriptyline, (4) amitriptyline, (5) p-
nitrophenol, (6) 5,5-diphenylhydantoin, (7) acetophenone, (8) benzonitrile, (9) 5-phenylpentanol, (10) anisole,
(11) 4-n-butylbenzoic acid, (12) toluene, (13) cis-chalcone, (14) trans-chalcone, (15) mefenamic acid, and (16)
ethylbenzene.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Researchers used gas chromatography to determine the % v/v methyl salicylate in rubbing alcohol [Van Atta, R. E.; Van Atta, R. L. J. Chem. Educ. 1980, 57, 230-231]. A set of standard additions was prepared by transferring 20.00 mL of rubbing alcohol to separate 25 - mL volumetric flasks and pipeting 0.00 mL, 0.20 mL, and 0.50 mL of methyl salicylate to the flasks. All three flasks were diluted to volume using isopropanol. Analysis of the three samples gave peak heights for methyl salicylate of 57.00 mm, 88.5 mm, and 132.5 mm, respectively. Determine the % v/v methyl salicylate in the rubbing alcohol.arrow_forwardPlease don't provide handwritten solution ......arrow_forward4.Consider the peaks for pentafluorobenzene and benzene in the gas chromatogram shown here. The elution time for unretained solute is 1.06 min. The open tubular column is 30.0 m in length and 0.530 mm in diameter, with a layer of stationary phase 3.0 μm thick on the inner wall. a) Measruing the width, w, at the baseline on the chromatogram, find the number of plates for these two compounds b) Use your answer to (a) to find the resolution between the two peaks c) Using the number of plates N=sqrtN1*N2 with the values from (a) calcuate what the resolution should be and compare your answer with the measured resolution in barrow_forward
- What mass of MgCl₂ 6H₂O is required to make a 0.200 dm³ stock solution containing 25.0 ppm Mg(II)? What volumes of this stock solution are required to make 10.0 cm³ standard solutions containing 5.00, 1.00 and 0.10 ppm Mg(II)?arrow_forwardAn open tubular column with a diameter of 215 um and a stationary phase thickness on the inner wall of 0.51 um passes unretained solute through in 79 s. A particular solute has a retetion time of 467 s. What is the distribution constant for this solute? Kp = What fraction of time does this solute spend in the stationary phase? ts !! ttotalarrow_forward4.columns with one million theoretical plates operating by slip flow. The figure shows the gradient separation of two forms of fluorescently labeled bovine serum albumin separated on a)50 x 2.1 mm UHPLC column of 1.7 µm C4 silica particles and b)10 mm x 75µm colloidal crystal column of 470 nm C4 silica nanoparticles. What are the main differences in the chromatogram? What is the reason for the difference? Why does the colloidal crystal column have such a high number of theoretical plates (N)? How is the problem of high pressures with very small particles overcome?arrow_forward
- calculate the kovats retention indices for solutes 1 and 2 from the data shown and assume tm=0.50 min (methane)arrow_forwardIn a reverse-phase separation, a solute was found to have a retention time of 19.6 min, while an unretained peak had a retention time of 1.45 min when the mobile phase was 20/80 v/v% methanol/water. (b) Calculate the k’ for the solute. (c) Calculate the new k’ if the mobile phase is changed to 60/40 methanol/water assuming identical void volume. (d) Calculate a solvent composition that would bring k’ from initial value to a value of 1.20 using tetrahydrofuran/water mobile phase rather than 20/80 v/v % methanol/water.arrow_forwardA 10-ml transfer pipet and a 200-ml volumetric flask (TC) are used to make 0.100 M and 0.0050 M solutions from a stock solution of 2.000(plus or minus) 0.005 M. Calculate the percent relative error for the three solutions if perfect technique is used. (See Garland Experiments in Physical Chemistry, 10th ed. p. 639-642) Report the two new molarititesarrow_forward
- Calculate the vapor pressure of propanol (b.p. 97.0 ∘C97.0 ∘C) at the gas chromatography column temperature of 95.0 ∘C95.0 ∘C using the form of the Clausius–Clapeyron equation shown ln(?1?2)=−(Δ?vap?)(1?1−1?2)ln(P1P2)=−(ΔHvapR)(1T1−1T2) where ?R is the ideal gas constant, Δ?vapΔHvap is the enthalpy of vaporization, ?1T1 and ?2T2 are two different temperatures, and ?1P1 and ?2P2 are the vapor pressures at the respective temperatures. The enthalpy of vaporization can be estimated using Trouton's rule, Δ?∘vap=(88 J·mol−1⋅K−1)×?bp.ΔHvap∘=(88 J·mol−1⋅K−1)×Tbp. ?=P= TorrTorr Calculate the vapor pressure of pentane (b.p. 36.0 ∘C36.0 ∘C) at the same column temperature. ?=P= TorrTorr For compounds of the same chemical class, what is the relationship between vapor pressure of a compound and retention in gas chromatography? As the vapor pressure decreases, retention time decreases. As the vapor pressure increases, retention time increases. As…arrow_forwardAnswer the questions by using the given graph belowarrow_forwardA solvent passes through a chromatography column in 3.87 min, but the solute requires 7.60 min. What is the retention factor, k? k = What fraction of the time does the solute spend in the mobile phase in the column? t fraction in mobile phase = The volume of the stationary phase is 0.133 times the volume of the mobile phase in the column (Vs = 0.133VM). What is the distribution constant, Kp, for this system? KD = %3Darrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning
