During the solution of an equilibrium problem you derive this expression: x² Kc = 3.7 x 10-10 (0.97 - x)² In this case, what is the 'x is small' assumption? (~ means 'approximately equal to") 01 0≈0 Or≈ Ke 0.97-xx 0.97 -≈ 0.97
During the solution of an equilibrium problem you derive this expression: x² Kc = 3.7 x 10-10 (0.97 - x)² In this case, what is the 'x is small' assumption? (~ means 'approximately equal to") 01 0≈0 Or≈ Ke 0.97-xx 0.97 -≈ 0.97
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter5: Introduction To Chemical Equilibrium
Section: Chapter Questions
Problem 5.41E: Write the equilibrium constant expression for each reaction in terms of activities, simplifying...
Related questions
Question
Please answer.
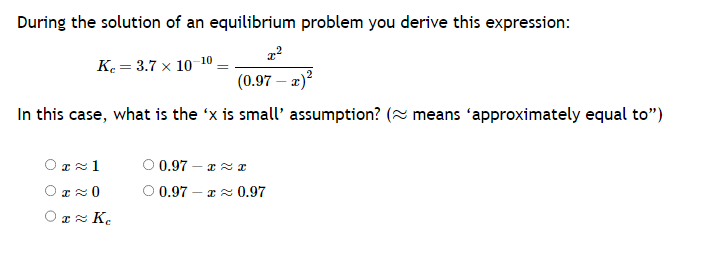
Transcribed Image Text:During the solution of an equilibrium problem you derive this expression:
x²
K₂= 3.7 x 10-10
(0.97 - x)²
In this case, what is the 'x is small' assumption? (~ means 'approximately equal to")
0≈1
OT≈ 0
01≈ Ke
O 0.972
0.97 -≈ 0.97
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning