e words in the left column to the appropriate blanks in the sentences on the right. Reset Hel are The for K+ is the potential difference across the cell membrane at which the inward potassium ion concentrations would be in equilibrium and there be no net flow across the membrane. weaker The is the actual measured potential difference. outward The two are not equal, so the potassium ion concentrations in equilibrium. stronger Nernst potential The membrane potential is negative, so the membrane electric field points are not The membrane potential is more negative than the Nernst potential, so the membrane electric field is membrane potential than needed to bring the net flow to zero. This electric field drives an ion flow that is larger than the diffuse flow due to the concentration gradient. Thus the net flow is
e words in the left column to the appropriate blanks in the sentences on the right. Reset Hel are The for K+ is the potential difference across the cell membrane at which the inward potassium ion concentrations would be in equilibrium and there be no net flow across the membrane. weaker The is the actual measured potential difference. outward The two are not equal, so the potassium ion concentrations in equilibrium. stronger Nernst potential The membrane potential is negative, so the membrane electric field points are not The membrane potential is more negative than the Nernst potential, so the membrane electric field is membrane potential than needed to bring the net flow to zero. This electric field drives an ion flow that is larger than the diffuse flow due to the concentration gradient. Thus the net flow is
Chapter18: Introduction To Electrochemistry
Section: Chapter Questions
Problem 18.4QAP
Related questions
Question

Transcribed Image Text:A cell's Nernst potential for K* ions is -60 mV. At
one instant of time, the cell's membrane potential is
-75 mV.
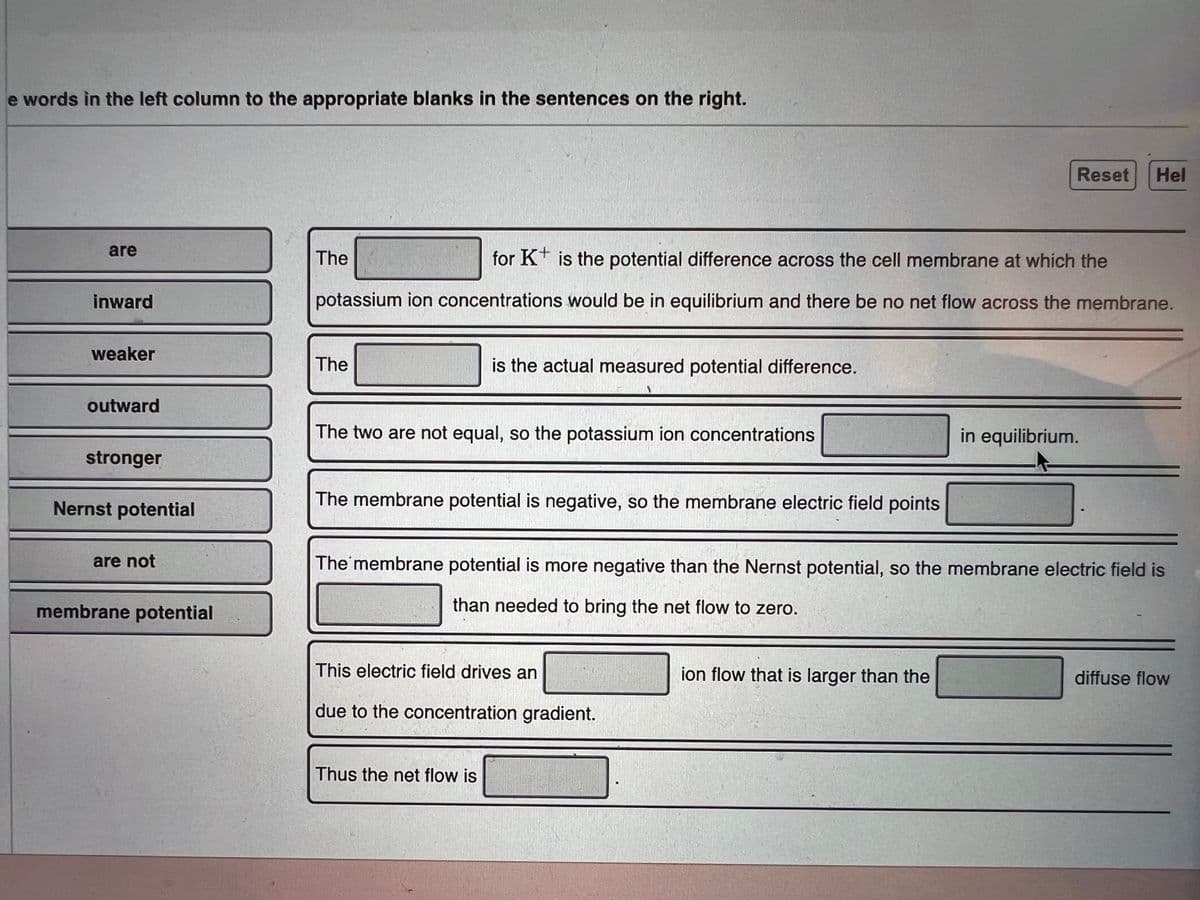
Transcribed Image Text:e words in the left column to the appropriate blanks in the sentences on the right.
Reset
Hel
are
The
for K* is the potential difference across the cell membrane at which the
inward
potassium ion concentrations would be in equilibrium and there be no net flow across the membrane.
weaker
The
is the actual measured potential difference.
outward
The two are not equal, so the potassium ion concentrations
in equilibrium.
stronger
Nernst potential
The membrane potential is negative, so the membrane electric field points
are not
The membrane potential is more negative than the Nernst potential, so the membrane electric field is
membrane potential
than needed to bring the net flow to zero.
This electric field drives an
ion flow that is larger than the
diffuse flow
due to the concentration gradient.
Thus the net flow is
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning
