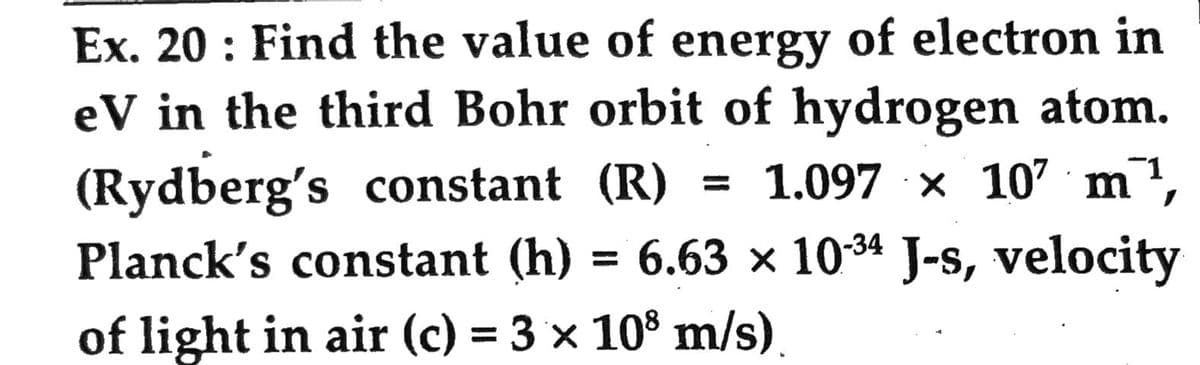
Ex. 20: Find the value of energy of electron in eV in the third Bohr orbit of hydrogen atom. (Rydberg's constant (R) 1.097 x 107 m¹, Planck's constant (h) = 6.63 × 10-³4 J-s, velocity of light in air (c) = 3 × 108 m/s)
Q: During working maintain 4 d.p. Round answer to whole numbers. What is the caloric expenditure for a…
A: Mass of the client, M=90 Kg time taken, T=10 minutes=10×60=600 s Power of the bicyclist, P=250 W…
Q: In the figure, two speakers separated by distance dy-120 m are in phase Assume the audes of the…
A: Since we answer up to three subparts, we will answer the first three. Please resubmit the question…
Q: Since Mars has an atmosphere and it is composed mostly of a greenhouse gas, why isn't there a…
A: The greenhouse effect is the absorption of radiation coming from the Sun by greenhouse gases. This…
Q: The z-transform of [n] = u[-n] is
A:
Q: A body of mass 2 kg was dropped down from a height. If the KE attained by it is 400 J, find the…
A: Solution:-Given thatmass of body (m)=2 kgKE attained by the mass (KE)=400 J
Q: We will assume that a sound wave, once generated and emitted, will propagate uniformly in all…
A:
Q: 8. Consider the system shown in figure. Block A weighs 45.0 N and block B weighs 25.0 N. Once block…
A: As, Wa=45 N So, mag=45ma=459.8ma=4.58 Kg Also, Wb=25 N So, mbg=25mb=259.8mb=2.55 Kg
Q: ound load (P = 12000 lbf). The support is required to be a hollow tube with a length of ! = 12 in.)…
A: crippling load refers to the maximum load at which the column experiences lateral displacement or…
Q: 1.5. A particle of mass m moves linearly under the action of a time varying force. The force time…
A: We are aware that a particle with mass m travels linearly when a time-varying force acts on it.The…
Q: The physical unit of kinetic energy is: - Kg-cm2/s2 - Gm/s2 -Gm-cm/s2. - None of the above
A: Option (A) Answer is explained below
Q: 5) If the two forces shown to the right each have magnitude 20.0 N, and the righthand force vector…
A: The magnitude of the two forces is given as, F1=F2=20 N. The angle of the forces is given as, θ=35o,…
Q: Jake pours 350 g of water at 41°C into a 750 g aluminum container that is at an initial temperature…
A: The water, initially at 41 C is poured into an aluminum container at a lower temperature of 12 C.…
Q: Read the question carefully before answering how complete solution with proper derivatives /…
A: Given: The three springs are in parallel. The height is h=1.5 m. The mass of the man is m=10 kg. The…
Q: You have 10 balls which are initially all on the left half of a box. A partition separating the two…
A: Given: Initial condition : All 10 balls on the left side. Final condition : 4 balls on left and 6…
Q: The strain components & & and Yxy are given for a point in a body subjected to plane strain.…
A: Solution:- Given; εx=-900 με εy=550 με yxy=1450 θ=35°…
Q: Max Plank brought the idea that energy is quantized. Explain how Plank's idea brought a perspective…
A: Maxwell’s equation wave nature of electromagnetic radiation was helpful in explaining phenomena such…
Q: Consider a cam follower machine be modeled as a mass-spring-damper system. The base of thi system is…
A: There is a Fourier series when the sines and cosines of a periodic function f(x) are added…
Q: If a hollow sphere of radius R is charged with one unit of static electricity, then the field…
A: Given: The electric field function. E(x)=0for 0<x<R12x2for x=R1x2for x>R
Q: Two coils close to each other have a mutual inductance of 29 mH. The current in one coil decays at…
A: Given that, L=29mH I=I0e-αt eqn 1here, I0=6 Athen,…
Q: In the laboratory frame, two observers A and B are moving along the sides of an equilateral triangle…
A:
Q: A 90 mH inductor carries a current that varies with time according to... ¡(t) = t²+6t (in A with t…
A: Given that-The inductance of the inductor, L= 90mHCurrent, it= t2+ 6tEMF will induce in the coil due…
Q: Read the question carefully before answering. Show complete solution with proper derivatives /…
A:
Q: charges and coordinates of two charged particles held fixed in an xy plane are q₁ -2.29 uc. x₁ -2.71…
A: The magnitude of electrostatic force on particle 2 due to particle 1 The distance between the…
Q: 1-A bubble of oil on the surface of the water, light incident at wavelength() at the angle 60°. if…
A: The incident light is what illuminates your scene. This is why it's a more accurate light reading…
Q: 2 What is the difference between SEM and TEM electron microscopes?
A:
Q: Which of these equations is correct (choose one only) ? O V2= A*V2 - B*12 O V1= A*V1 - B*12 O V1=…
A: Solution: According to the principle of homogeneity, the physical quantity having the same…
Q: Solving Poisson's equation V²p=-Po/&o for the electrostatic potential p(x) in a region with a con…
A:
Q: What is the current through the battery when the switch is open? 10 V 20 Ω 60 Ω ули 40 Ω 10 Ω
A:
Q: Do it please
A: Given that-Length of the vertebra, L0=0.7 cm=0.007 mDiameter of the vertebra, d=4 cm=0.04mShear…
Q: Equation of an alternating e.m.f. is given by e = 100 sin (100 t). Find the peak value of e.m.f. and…
A: To find-(1) Peak value of emf=?(2) Frequency of alternating emf=?Given-e=100 sin (100πt)Equation of…
Q: Example 3.3 Determine the angle of tilt required for a polar mount used with an earth station at…
A: The antenna is moved by a single actuator in a circular motion. These antennas often exhibit some…
Q: Calculate the Poisson's ratio for silver. Given Young's modulus for silver is 7.25 x 10¹⁰ N/m² and…
A: Poisson's ratio: Poisson's ratio is a dimensionless quantity, defined as the ratio of lateral strain…
Q: 2 Using The method of dimensions, derive an expression for rate of flow (4) of aliquid Through a…
A: The rate of flow of a liquid, Q, depends on radius of a pipe (r), pressure gradient, PL and on…
Q: 1.b. Four infinite uniform sheets of charge ax located as follows: h 20 PC/m² at y = 7 -8 PC/m² at y…
A: Given: The surface charge densities of the uniform plane sheet are σ1 =-18 pC/m2 at y = -4σ2 = 6…
Q: What is the effect of the mass of a pendulum on its period? O The period is unaffected The period is…
A: Given that-Time period of a pendulum, T=2π×lg equation 01In above formula, there is no mass(m)
Q: 1 2 3 4 What is the difference between the "top to down" and "bottom to up" approach for synthesis…
A: Disclaimer: “Since you have asked multiple question, we will solve the first question for you. If…
Q: A sinusoidal transverse wave is traveling along a string in the negative direction of the x-axis.…
A:
Q: When heat flows into a diatomic ideal gas, the pressure is constant and the volume increased. Find…
A: Concept: In this question, we have to calculate the fraction of heat that becomes the work for the…
Q: (b) A grindstone in the shape of a solid disk with diameter 0.520 m and a mass of 50.0 kg is…
A: Given that-d=0.520 mm=50 kgω0= 850 rev/minF=160 NInitial Angular velocity, ω0= 850…
Q: What is the reactance is 10 at 60 Hz? self-inductance of a coil whose
A: Given that-The reactance of the inductor is 10ΩOperating frequency is 60 HzWe know…
Q: Most spectroscopic analyzes are made of: - chrome - quartz - Ordinary glass - pyrex glass
A: The problem is based on spectroscopic techniques. Different materials are used in a spectrograph…
Q: A 5.22-kg object passes through the origin at time t=0 such that its x component of velocity is 4.70…
A:
Q: 1/3 @a P2 ア P= Pi
A:
Q: F5
A: When the switch is open the resistance 20Ω and 60Ω are in seriesand, 40Ω and 10Ω are in series.Here,…
Q: Find the direction and the value of current for the circuit shown below 12.0 www .6.7V 90 28V
A: Given: circuit diagram with 12, 9 ohms resistor.and batteries of voltage 28V ,6.7V with si and Ge…
Q: 9) A spring has a constant of 875 N/m. What hanging mass will cause this spring to stretch 4.5 m?
A: Let's Consider a vertical spring of spring constant 'k' On which we hang a mass 'm'It will stretch a…
Q: The GaAs sample at T= 300 K with doping concentrations of [N₂ =0 and N= 10¹6 cm-³]. Assume complete…
A:
Q: y_large (cm) 100 50 -50 О 0.1 0.2 0.3 0.4 0.5 0.6 0.7
A:
Q: 6
A: Given that,Mass of railway train : m1=6000 kgVelocity of railway train : V1=2.0 m/s RightMass of…
Q: Q5: a) Find the magnetic vector potential A of an infinite solenoid with n turns per unit length,…
A:
Trending now
This is a popular solution!
Step by step
Solved in 2 steps
