
Fundamentals Of Analytical Chemistry
9th Edition
ISBN: 9781285640686
Author: Skoog
Publisher: Cengage
expand_more
expand_more
format_list_bulleted
Question
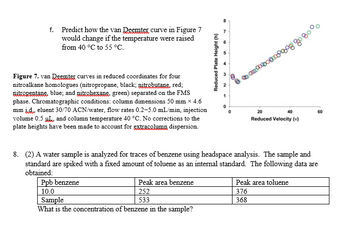
Transcribed Image Text:f. Predict how the van Deemter curve in Figure 7
would change if the temperature were raised
from 40 °C to 55 °C.
Figure 7. van Desmter curves in reduced coordinates for four
nitroalkane homologues (nitropropane, black; nitrobutane, red;
nitropentane, blue; and nitrohexane, green) separated on the FMS
phase. Chromatographic conditions: column dimensions 50 mm × 4.6
mm id, eluent 30/70 ACN/water, flow rates 0.2-5.0 mL/min, injection
volume 0.5 and column temperature 40 °C. No corrections to the
plate heights have been made to account for extracolumn dispersion.
Reduced Plate Height (h)
°
20
40
60
Reduced Velocity (v)
8. (2) A water sample is analyzed for traces of benzene using headspace analysis. The sample and
standard are spiked with a fixed amount of toluene as an internal standard. The following data are
obtained:
Ppb benzene
Peak area benzene
Peak area toluene
10.0
252
376
Sample
533
368
What is the concentration of benzene in the sample?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 3 images

Knowledge Booster
Similar questions
- When naphthalene, butyric acid, and phenyl acetate are separated using dichloromethane as the developing solvent on a silica gel TLC plate, which compound would have the highest Rf value? Which would have the lowest?arrow_forward2. Thin layer chromatography was performed to find the most effective mobile phase needed to separate a mixture containing three compounds (using silica gel as the stationary phase). TLC analyses were performed using five different mobile phases: (A) pure petroleum ether; (B) 25:75 ethyl acetate:petroleum ether; (c) 50:50 ethyl acetate:petroleum ether (D) 75:25 ethyl acetate:petroleum ether; and (E) pure ethyl acetate. Using the TLC results provided, indicate whether an effective separation was achieved for each of the five mobile phases. Be sure to provide a brief explanation for your answer and identify which mobile phase(s) could be used to offer an effective separation of this mixture using column chromatography. A D Earrow_forwardA mixture composed of biphenyl, benzoic acid, and benzyl alcohol is spotted on a silica gel TLC plate and eluted with a 10:1 hexanes:ethyl acetate solvent mixture. In what order are the Rf values of the 3 compounds (lowest first)?arrow_forward
- Would you expect an alcohol or an ether to have a higher Rf value on silica gel thin layer chromatography? Why?arrow_forwardDifference between Experimental IR and literature IR of pure salicylic acid Explanation on the different regions found on an IR spectrum (diagnostic vs fingerprint)- Explanation on how chemical effects (for example, the conjugation of C=O with C=C double bond) alter peak regions.- Comparison of specific functional group diagnostic peaks (C=O carboxylic acid, C-H, O-H carboxylic acid, O-H phenol, C=C) between Literature and Experimental spectra.- Comparison of fingerprint region between literature and experimental spectra.arrow_forwardNumber 2arrow_forward
- A mixture of p-xylene, m-xylene and o-cresol was injected into a gas chromatograph. Unretained o-crosol afforded a sharp spike at 59 s, whereas p-xylene required 247 s and m-xylene was eluted in 357 s. Find the relative retention for the p- and m- isomers of xylene.arrow_forwardB1arrow_forward1) A mixture of benzoic acid, para-xylene and benzaldehyde is spotted on a silica gel TLC plate and developed using Ethyl Acetate with 0.5% acetic acid the solvent. Predict the relative Re values for the three components in the sample (highest to lowest). 2) Arrange the following solvents in order of increasing polarity (least polar to most polar): Diethyl Ether, Propyl Amine, 3-Methyl-Pentane, Acetonearrow_forward
- f. What is the order of elution for the following compounds from a reversed-phase LC column? Hint: Rank the compounds by their polarity. i. benzene diethyl ether n-hexane ii. CIH2C-CH2CI NH2 acetone dichloroethane acetamidearrow_forwardis hexane or 70:30 hexane: ethyl acetate a better eluent based on their TLC plates and rf value in column chromatography separation of ferrocene-acetylferrocene.arrow_forwardCis-3-hexene and trans-3-hexene can be separated and analyzed using GC without injecting an equimolar standard to a high accuracy. Explain why that is the case for these two compounds while we had to inject an equimolar solution for our experimentarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole