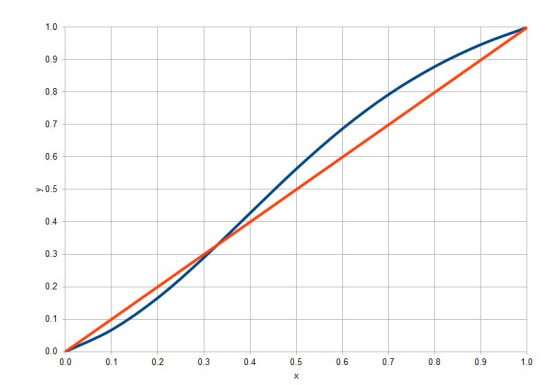
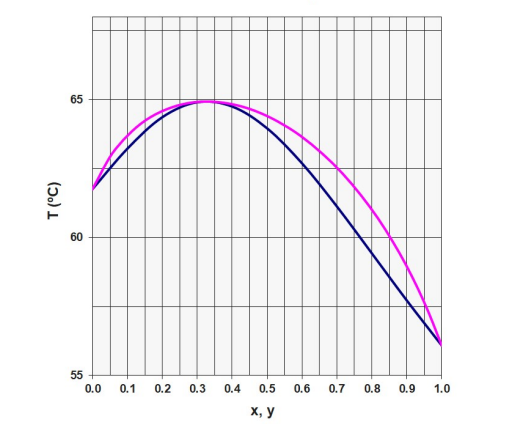
Given the Macabe-Thiele diagram for the acetone-chloroform system (on a molar basis and written for acetone) and also the Txy diagram, solve the following problem considering the Lewis assumptions for the distillation column. Also use the molar mass of 58.1 g/mol for acetone and 119.4 g/mol for chloroform. An acetone-chloroform binary solution with 70% by mol of acetone will be distilled in a column of dishes. The liquid solution in question will be preheated to something around saturation under column operating conditions. the flow of the mixture was estimated at 2 kmol/h to meet a certain production demand. Top product with 95% acetone on a molar basis and bottom product with 55% by mol chloroform is desired. a) How much will the top and bottom production be (in molar flows)? What will the acetone recovery be? (Answer: 1 kmol/h of bottom and top product, with 68% recovery for acetone) b) Using a reflux ratio of 2.4, calculate the number of equilibrium stages of the distillation column. How many ideal dishes were estimated assuming total condenser? c) Assuming total condenser, what would be the ideal temperature in the first plate (top plate) according to the estimate based on the equilibrium of each stage? Given the same assumptions, what would be the temperature of the feeding plate? d) Would it be possible to use a column for binary distillation described in the problem, but replacing the specification of the bottom with chloroform 90 % in mol?
Given the Macabe-Thiele diagram for the acetone-chloroform system (on a molar basis and written for acetone) and also the Txy diagram, solve the following problem considering the Lewis assumptions for the distillation column. Also use the
molar mass of 58.1 g/mol for acetone and 119.4 g/mol for chloroform.
An acetone-chloroform binary solution with 70% by mol of acetone will be distilled in a column of dishes. The liquid solution in question will be preheated to something around saturation under column operating conditions. the flow
of the mixture was estimated at 2 kmol/h to meet a certain production demand. Top product with 95% acetone on a molar basis and bottom product with 55% by mol chloroform is desired.
a) How much will the top and bottom production be (in molar flows)? What will the acetone recovery be? (Answer: 1 kmol/h of bottom and top product, with
68% recovery for acetone)
b) Using a reflux ratio of 2.4, calculate the number of equilibrium stages of the distillation column. How many ideal dishes were estimated assuming total condenser?
c) Assuming total condenser, what would be the ideal temperature in the first plate (top plate) according to the estimate based on the equilibrium of each stage? Given the same assumptions, what would be the temperature of the feeding plate?
d) Would it be possible to use a column for binary distillation described in the problem, but replacing the specification of the bottom with chloroform 90 % in mol?
Step by step
Solved in 4 steps with 4 images









