
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
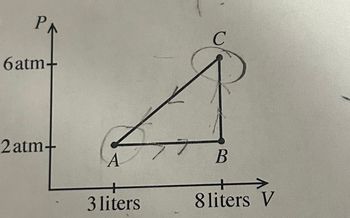
Given the PV diagram of a fixed quanity of CO2 assumed to be an ideal gas. The temperature at point a A is 35 degrees celsius. What is the total mass of CO2? What ist he temperature at point C in degrees celsius? What is the heat added to the gas over the ABCA cycle? If 3200 J of heat flows out of the system when the system is taken in a straight path from C to A, what is the change in enteral energy along that path?
Transcribed Image Text:PA
6atm+
2 atm+
2
A
3 liters
C
B
8 liters V
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Step 1: Determine the given variables.
VIEW Step 2: Part (i) - Determine the total mass of the gas
VIEW Step 3: Part (ii) - Determine the temperature at C in degree celsius
VIEW Step 4: Part (iii) - Determine the heat added to the gas in a complete cycle.
VIEW Step 5: Part (iv) - Determine the change in internal energy for C to A
VIEW Solution
VIEW Trending nowThis is a popular solution!
Step by stepSolved in 6 steps with 6 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- How much heat does it take to increase the temperature of 2.00 moles of an … Part A: ideal monatomic gas from 17 degrees C to 37 degrees C if the gas is held at constant volume? Part B: ideal diatomic gas from 17 degrees C to 37 degrees C if the gas is held at constant volume? (express both answers in joules)arrow_forwardPart A: 10 moles of ideal gas initially at 10 atm and 250 K is expanded isothermally and reversibly to a final pressure of 2 atm. Calculate W, AU, and Q in Joules. Part B: The same gas in problem A, starting in the same initial state, is expanded isothermally against a constant external pressure of 2 atm, to a final pressure of 2 atm. Calculate W, AU, and Q in Joules for this irreversible process. Part C: The same gas in part B, starting in the same initial state, is expanded isothermally to a final pressure of 2 atm. The process is carried out in 4 stages. First the gas is expanded against a constant pressure of 8 atm, to 8 atm. Then the gas is expanded against a constant pressure of 6 atm, to 6 atm. Then the gas is expanded against a constant pressure of 4 atm, to 4 atm. Finally, the gas is expanded against a constant pressure of 2 atm, to 2 atm. Calculate W, AU, and Q in Joules for the 4-step process.arrow_forwardI need the answers for d-f but please also answer a-c as well.arrow_forward
- How to solve this questionarrow_forwardAn ideal gas is confined to a constant volume container and heated from a pressure of 1 atm at room temperature to a pressure of 5 atm. What is the final temperature. Thank you for the help. I got 297K but I do not think that is it.arrow_forwardIf we know that the internal energy of the monoatomic ideal gas remains constant during the process in B to C, what must be the pressure Pc at point C in terms of the original pressure P0.arrow_forward
- This is a calculation only. Energy2d isn’t able to do phase changes as far as I’ve learned. There was a different simulation for this but it is not currently free. You combine 50g of ice at -10oC with 150g of liquid water at 75oC. After a few minutes, you measure the final equilibrium temperature of all of the H2O to be 35.1 oC. Use this information to “experimentally” determine the heat of transformation of water, Lfusion. cwater=4190 J/kgK, cice=2090 J/kgK Show all of your work and compare to the accepted value Lfusion=3.33x105 J/kg.arrow_forwardA flexible box contains 5.60 grams of nitrogen gas (N2) which is maintained at a constant pressure of 1.35 x 10$ Pa. The box is placed over a fire, causing the volume to increase from 0.00200 m3 to 0.00300 m³. Find the increase in temperature of the gas. (For N2 molar mass M = 28 grams.)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON