
Appl Of Ms Excel In Analytical Chemistry
2nd Edition
ISBN: 9781285686691
Author: Crouch
Publisher: Cengage
expand_more
expand_more
format_list_bulleted
Question
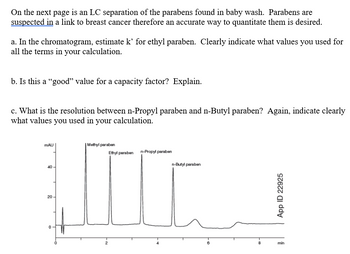
Transcribed Image Text:On the next page is an LC separation of the parabens found in baby wash. Parabens are
suspected in a link to breast cancer therefore an accurate way to quantitate them is desired.
a. In the chromatogram, estimate k' for ethyl paraben. Clearly indicate what values you used for
all the terms in your calculation.
b. Is this a "good" value for a capacity factor? Explain.
c. What is the resolution between n-Propyl paraben and n-Butyl paraben? Again, indicate clearly
what values you used in your calculation.
MAU
| Methyl paraben
40
20
0
-2
Ethyl paraben n-Propyl paraben
n-Butyl paraben
App ID 22925
6
8
min
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- 16. In this chromatographic technique the mobile should consists of buffer solution or with known high ionic strength A. GC-MS B. RP-HPLC C. IEC D. NP-HPLCarrow_forwardI) Choose the Correct Answer from the Multiple-Choice List (10) 1. Which of the following is not a common type of packing for reversed-phase HPLC? C. Diol D. phenyl A. C18 B. C8 2. Gradient elution is a separation that employs : a. a single solvent of constant composition. b. solvent mixture of constant composition. c. two or more solvent systems that differ significantly in polarity. d. both (a) and (b). 3. The most useful bonded-phase coatings in HPLC are . : a) glass b) alumina c) inert silica d) siloxanes 4.Which of the following solvents is not commonly used in reversed-phase HPLC? a) Ethanol Water b) Hexane c) Acetonitrile d) Methanol e) water 5. It is not of the solute property detector used in HPLC is: a. Refractive index detector b. UV detector c. fluorescence detector d. UV-visible detector 6. High performance liquid chromatography (HPLC) can be used to : a. identify the various pigments from a leaf extract. b. separate types of organic pesticides. c. determine the mercury…arrow_forward1) Study the chromatograph (below) of a mixture of Compounds A and B, run on the GCs in the teaching labs at CU Boulder. Compound A has the shorter retention time. STAAT 61 1.11 227 RT TYPE AREA XXXX XXXX XXXX AREAS 0.009 55874 44.117 ARIHT 0.61 XX XX XX 1.11 2.27 XX XX XX TOTAL AREA=XX MUL FACTOR=XX 1. What is the retention time of compound A? Compound B? 2. Which compound is present in a larger amount? 3. Which compound has the lower boiling point? 4. What would happen to the retention times of compounds A and B if the column temperature were raised? 5. You suspect that compound B is octane. What can you do to provide supporting evidence for this hypothesis?arrow_forward
- 7. In gas chromatography, increasing the column length leads to analytes, assuming all other parameters are kept constant. a. no effect on b. an increase in C. a decrease in 8. Suppose you are extracting an organic compound from an aqueous solution using retention time for dichloromethane. a. top layer b. bottom layer Once you mix the two layers together, which layer is the organic extract? 6001 9. Which way is best to remove an acidic impurity from an organic liquid that is immiscible with water? a. simple distillation b. wash with aqueous base c. wash with aqueous acid d. recrystallization it in diegolved in a volatilearrow_forwardThin Layer Chromatography. All types of chromatography have mobile phaseand stationary phase. What is a stationary phase and a mobile phase? Elaborate your answer.arrow_forwardplease solve parts d and e and f, last three parts and thanks alotarrow_forward
- Which one is the more polar component? Explain.arrow_forwardكرة HPLC Q3: The Figure shown a HPLC chromatogram of two compound .Calculate the following values using the data in this chromatogram. Detector signal a. The adjusted retention time of uracil and guanine. b. The peak widths for uracil and guanine. The retention factor for uracil and guanine. C. d. The selectivity factor for uracil and guanine. e. The resolution for these two compounds. f. The column efficiency parameters, N and H, if the column length is 10cm. 0.6- 05 04 03 0.2 0.1- 45.0 s vold A 15 30 45 تختبر تحليل الى 180.0 s Auracil 90 60 76 2475s guanine 105-120 135 150 166 180 195 210 225 240 255 270 296 Time, secondsarrow_forwardThe surface of the silica gel has a structure that looks like this diagram below. Suppose you used a plate coated with silica gel, with propanone, CH3COCH3, as the solvent for thin-layer chromatography. Suppose also that the mixture you were trying to identify contained benzaldehyde benzanilide phenol 1) rank the Rf value from large to small 2) name the IMF between each compound and the silica gel OH OH OH -0-Si-O-Si-O-Si-O- O main body of silica structurearrow_forward
- Sketch a chromatogram for a compound with a stronger interaction with the stationary phase than the one shown below (draw on top of the shown chromatogram). Briefly explain how and why it is different.arrow_forwardFor quantitative analysis with gas chromatography: O the area of a peak reported by the instrument for a compound is proportional to the quantity of that compound. O the calculated response factor value for a compound is proportional to the quantity of that compound. O the retention time reported by the instrument for a compound is proportional to the quantity of that compound. O the peak height reported by the instrument for a compound is inversely proportional to the quantity of that compound. O the peak shape reported by the instrument for a compound is proportional to the quantity of that compound.arrow_forwardWhich of the following compounds will elute LAST in a reverse phase chromatography? а. b. Br с. NH2 d.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemical Principles in the LaboratoryChemistryISBN:9781305264434Author:Emil Slowinski, Wayne C. Wolsey, Robert RossiPublisher:Brooks Cole
Chemical Principles in the LaboratoryChemistryISBN:9781305264434Author:Emil Slowinski, Wayne C. Wolsey, Robert RossiPublisher:Brooks Cole EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning


Chemical Principles in the Laboratory
Chemistry
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Brooks Cole


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning