Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter10: Energy
Section: Chapter Questions
Problem 38QAP: In the “Chemistry in Focus” segment Firewalking: Magic or Science?, it is claimed that one reason...
Related questions
Question
Answer number 10
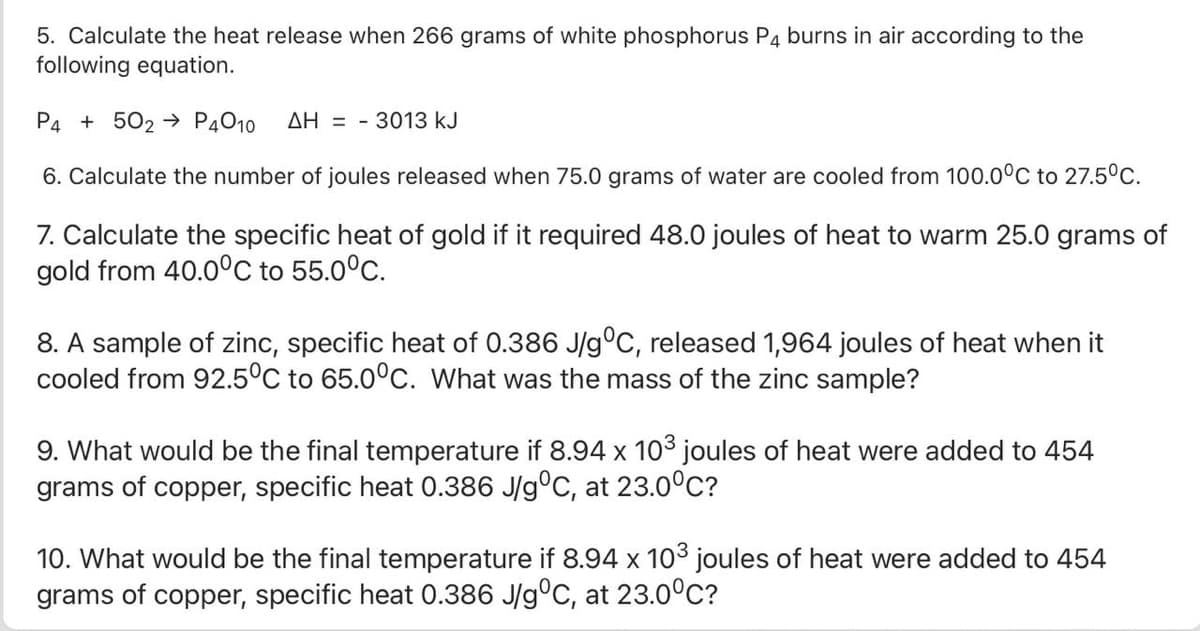
Transcribed Image Text:5. Calculate the heat release when 266 grams of white phosphorus P4 burns in air according to the
following equation.
P4 + 502 → P4010
AH = - 3013 kJ
6. Calculate the number of joules released when 75.0 grams of water are cooled from 100.0°C to 27.5°C.
7. Calculate the specific heat of gold if it required 48.0 joules of heat to warm 25.0 grams of
gold from 40.0°C to 55.0°C.
8. A sample of zinc, specific heat of 0.386 J/g°C, released 1,964 joules of heat when it
cooled from 92.5°C to 65.0°c. What was the mass of the zinc sample?
9. What would be the final temperature if 8.94 x 103 joules of heat were added to 454
grams of copper, specific heat 0.386 J/g°C, at 23.0°C?
10. What would be the final temperature if 8.94 x 103 joules of heat were added to 454
grams of copper, specific heat 0.386 J/g°C, at 23.0°C?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning