Phosphate buffered saline (PBS) is routinely used in the lab to wash cells that are being grown in dishes. Assuming intracellular concentrations are given in the table down below, calculate the Nernst Potential for Na+ and K+ of cells being washed by PBS.
Phosphate buffered saline (PBS) is routinely used in the lab to wash cells that are being grown in dishes. Assuming intracellular concentrations are given in the table down below, calculate the Nernst Potential for Na+ and K+ of cells being washed by PBS.
Chapter16: Applications Of Neutralization Titrations
Section: Chapter Questions
Problem 16.8QAP
Related questions
Question
Phosphate buffered saline (PBS) is routinely used in the lab to wash cells that are
being grown in dishes. Assuming intracellular concentrations are given in the table down below,
calculate the Nernst Potential for Na+ and K+ of cells being washed by PBS. The recipe for
phosphate buffered saline is given below. (Note, for calcium chloride and magnesium chloride,
the amounts are listed as the hydrated salt. This means that for every unit of chloride salt, there
is associated water – two water molecules for calcium chloride and six for magnesium chloride –
that should be accounted for in the formula weight of the salt.
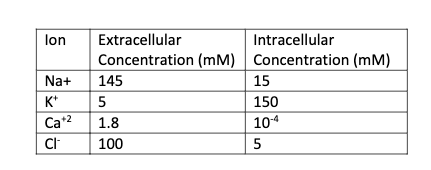
Transcribed Image Text:lon
Extracellular
Intracellular
Concentration (mM) Concentration (mM)
145
Na+
15
K*
5
150
Ca+2
1.8
104
100
5
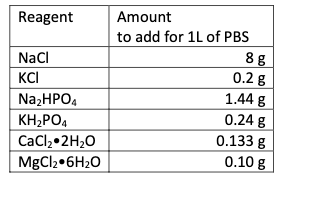
Transcribed Image Text:Reagent
Amount
to add for 1L of PBS
NaCl
8 g
0.2 g
1.44 g
KCI
NazHPO4
0.24 g
ΚHΡΟ.
CaCl,•2H2O
0.133 g
MgCl2•6H2O
0.10 g
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax