Please identify compound 1 and compound 2!!! Attached is the H-NMR and IR for both compounds (ignore the IR peaks ~2400). The experimental melting point for the aqueous layer was 260-261 celcius. The experimental melting point for the organic layer was 76.9-78.7 celcius. The following was my procedure: Introduction The reaction mixture in this experiment contains 4-bromobenzaldehyde, methanol, and aqueous potassium hydroxide. A reaction occurs that produces two organic compounds, compound 1 and compound 2. Both are solids at room temperature. Your task is to isolate, purify, and identify both compounds. A specific procedure is given for preparing the compounds, but you will need to work out the procedures for most other parts of this experiment. Experimental Procedure This procedure should produce enough of each compound to complete the experiment; however, in some cases, it may be necessary to run the reaction a second time. Although this experiment can be done individually, it works especially well for two students to work together. Add 1.50 g of 4-bromobenzaldehyde and 4.0 mL of methanol to a 25-mL round-bottom flask. With gentle swirling, add 4.0 mL of an aqueous potassium hydroxide solution with a Pasteur pipet. Avoid getting potassium hydroxide solution on the ground-glass joint! Add a stir bar to the flask and attach a water-cooled condenser. Using a hot-water bath, heat the reaction mixture - at about 65°C with stirring for 1 hour. Cool the mixture to room temperature, and add 10 mL of water to the flask. Pour the mixture into a beaker, and use another 10 mL of water to aid the transfer into the beaker. Using a separatory funnel, extract the reaction mixture with 10 mL of methylene chloride. Drain the organic layer (bottom) into another container. Extract the aqueous layer with another 10-mL portion of methylene chloride. Combine the organic layers. The organic layer contains compound 1, and the aqueous layer contains compound 2. Organic Layer Wash the organic layer two times with 10-mL portions of 5% aqueous sodium bicarbonate solution. Then, wash the organic layer with an equal volume of water. If an emulsion forms, use a little saturated sodium chloride solution to break it. Dry the organic layer over granular anhydrous sodium sulfate for 10-15 minutes. After the dried solution is removed from the drying agent, the organic layer should contain only compound 1 and methylene chloride. Isolate compound 1 by removing the methylene chloride on the rotovap. Purity compound 1 by crystallization. Use xylene to crystallize the compound using a hot-water bath at about 70°C, to avoid melting the sold. Aqueous Layer To precipitate compound 2, add 10 mL of cold water and acidify with 6 M HC. As acid is added, stir the mixture. Do not overacidity the solution; pH 3 or 4 is fine. if no precipitate is formed on acidification, add saturated NaCl to aid the process. This is called "salting out." Isolate compound 2, and dry it in an oven at about 110°C. Purify it by crystallization. Use methanol to purify the compound by crystallization.
Please identify compound 1 and compound 2!!!
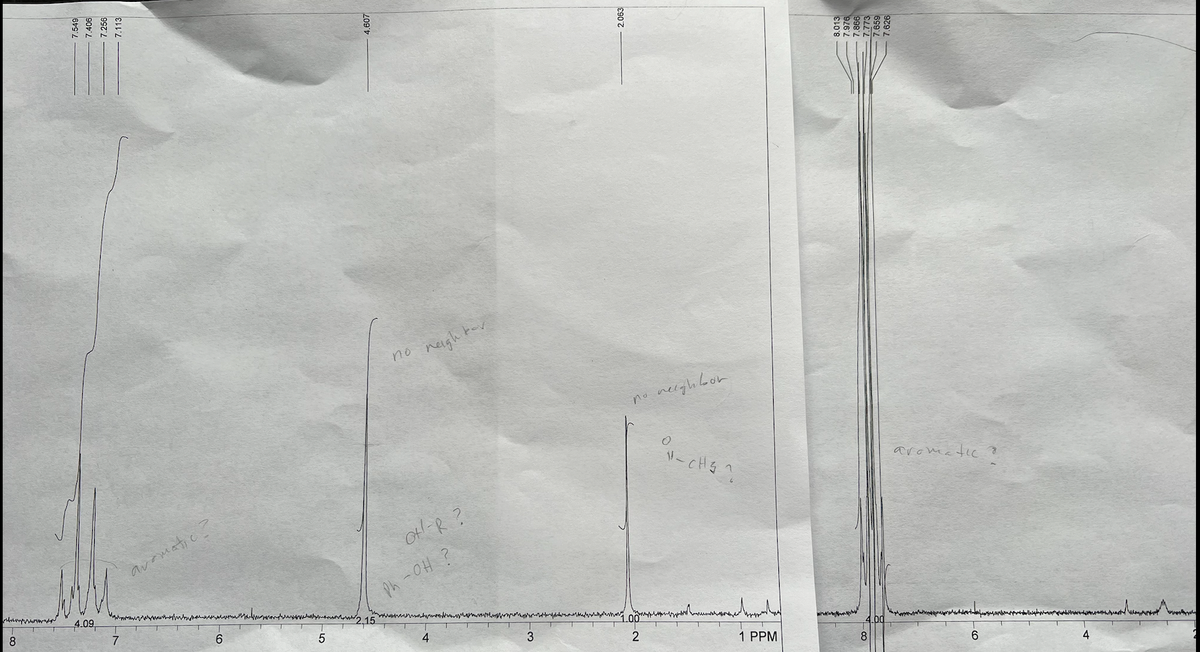
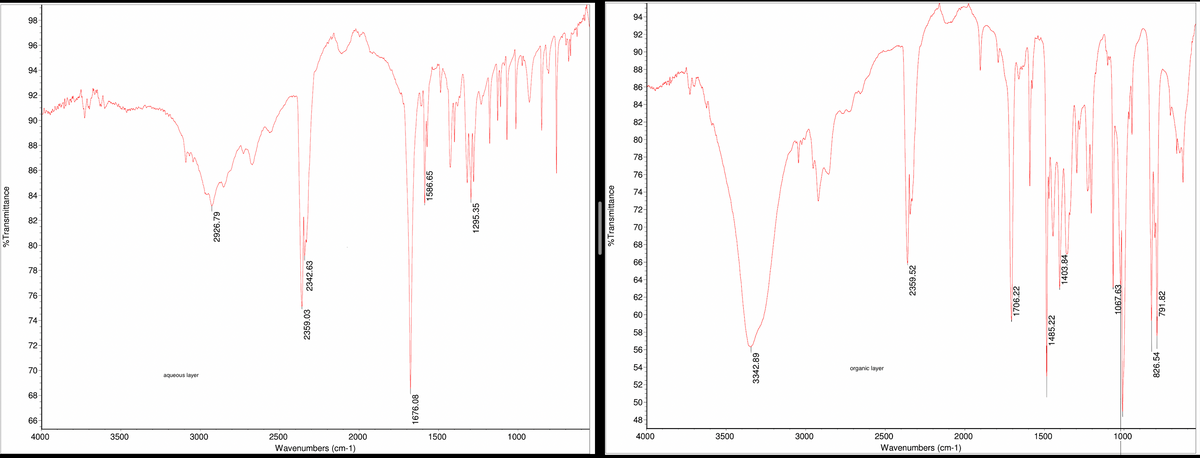
Attached is the H-NMR and IR for both compounds (ignore the IR peaks ~2400).
The experimental melting point for the aqueous layer was 260-261 celcius. The experimental melting point for the organic layer was 76.9-78.7 celcius.
The following was my procedure:
Introduction
The reaction mixture in this experiment contains 4-bromobenzaldehyde, methanol, and aqueous potassium hydroxide. A reaction occurs that produces two organic compounds, compound 1 and compound 2. Both are solids at room temperature. Your task is to isolate, purify, and identify both compounds. A specific procedure is given for preparing the compounds, but you will need to work out the procedures for most other parts of this experiment.
Experimental Procedure
This procedure should produce enough of each compound to complete the experiment; however, in some cases, it may be necessary to run the reaction a second time. Although this experiment can be done individually, it works especially well for two students to work together.
Add 1.50 g of 4-bromobenzaldehyde and 4.0 mL of methanol to a 25-mL round-bottom flask.
With gentle swirling, add 4.0 mL of an aqueous potassium hydroxide solution with a Pasteur pipet. Avoid getting potassium hydroxide solution on the ground-glass joint! Add a stir bar to the flask and attach a water-cooled condenser. Using a hot-water bath, heat the reaction mixture
- at about 65°C with stirring for 1 hour. Cool the mixture to room temperature, and add 10 mL of water to the flask. Pour the mixture into a beaker, and use another 10 mL of water to aid the transfer into the beaker.
Using a separatory funnel, extract the reaction mixture with 10 mL of methylene chloride. Drain the organic layer (bottom) into another container. Extract the aqueous layer with another 10-mL portion of methylene chloride. Combine the organic layers. The organic layer contains compound 1, and the aqueous layer contains compound 2.
Organic Layer
Wash the organic layer two times with 10-mL portions of 5% aqueous sodium bicarbonate solution. Then, wash the organic layer with an equal volume of water. If an emulsion forms, use a little saturated sodium chloride solution to break it. Dry the organic layer over granular anhydrous sodium sulfate for 10-15 minutes. After the dried solution is removed from the drying agent, the organic layer should contain only compound 1 and methylene chloride. Isolate compound 1 by removing the methylene chloride on the rotovap.
Purity compound 1 by crystallization. Use xylene to crystallize the compound using a hot-water bath at about 70°C, to avoid melting the sold.
Aqueous Layer
To precipitate compound 2, add 10 mL of cold water and acidify with 6 M HC. As acid is added, stir the mixture. Do not overacidity the solution; pH 3 or 4 is fine. if no precipitate is formed on acidification, add saturated NaCl to aid the process. This is called "salting out." Isolate compound 2, and dry it in an oven at about 110°C. Purify it by crystallization. Use methanol to purify the compound by crystallization.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images









