Question 22 Antimony has 2 stable isotopes 121-Sb mass 122.9042 amu. Calculate the percent abund Hint: Use amu from periodic table and assign for one isotope and 1-x value for other to sol O 57% and 43% O 50% and 50% O 80% and 20% 40% and 60%
Question 22 Antimony has 2 stable isotopes 121-Sb mass 122.9042 amu. Calculate the percent abund Hint: Use amu from periodic table and assign for one isotope and 1-x value for other to sol O 57% and 43% O 50% and 50% O 80% and 20% 40% and 60%
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter2: Atoms Molecules And Ions
Section2.2: Isotopes And Atomic Weight
Problem 2.3CYU: Neon has three stable isotopes, one with a small abundance. What are the abundances of the other two...
Related questions
Concept explainers
Atomic Structure
The basic structure of an atom is defined as the component-level of atomic structure of an atom. Precisely speaking an atom consists of three major subatomic particles which are protons, neutrons, and electrons. Many theories have been stated for explaining the structure of an atom.
Shape of the D Orbital
Shapes of orbitals are an approximate representation of boundaries in space for finding electrons occupied in that respective orbital. D orbitals are known to have a clover leaf shape or dumbbell inside where electrons can be found.
Question
100%
Both answer please...
So, kindly upvote you.....
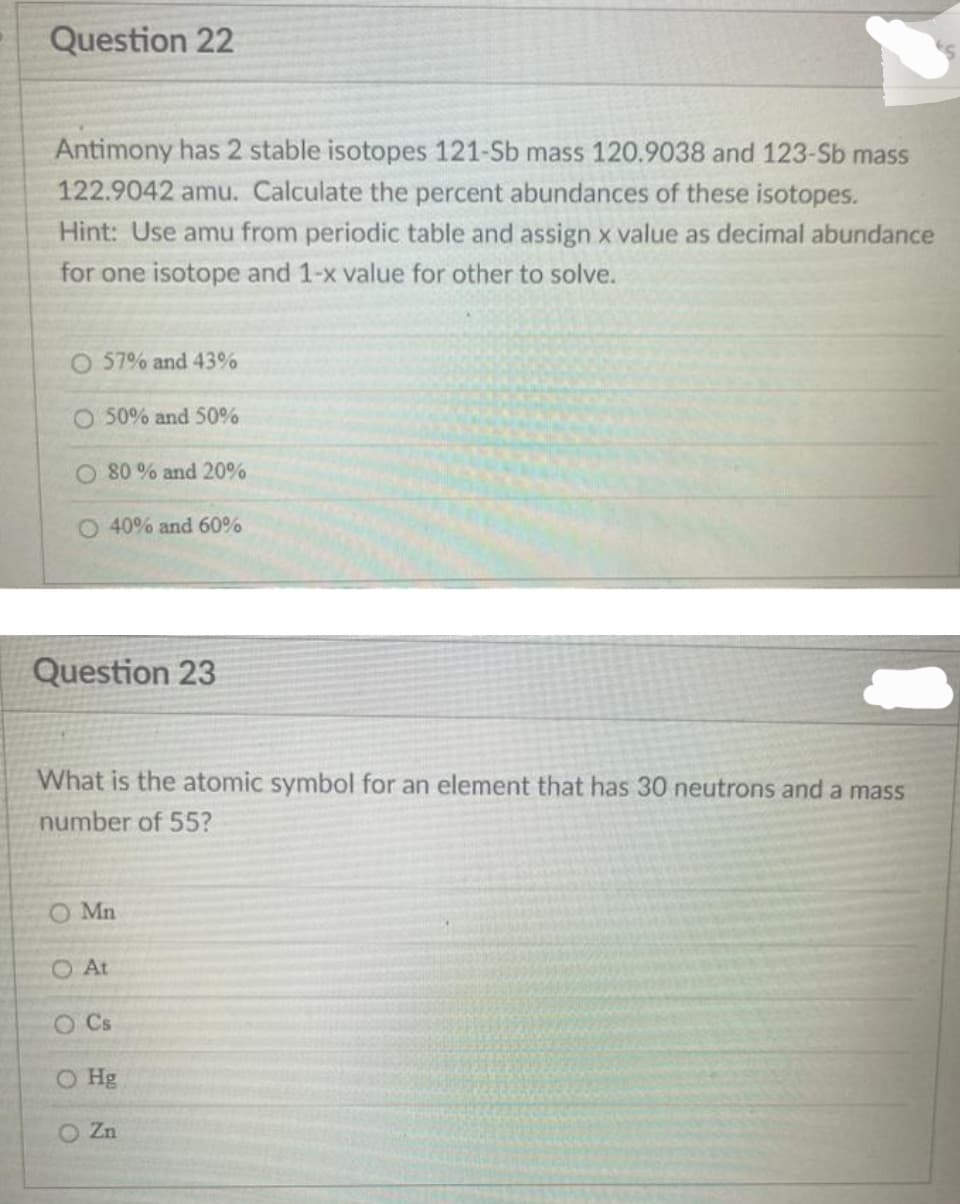
Transcribed Image Text:Question 22
Antimony has 2 stable isotopes 121-Sb mass 120.9038 and 123-Sb mass
122.9042 amu. Calculate the percent abundances of these isotopes.
Hint: Use amu from periodic table and assign x value as decimal abundance
for one isotope and 1-x value for other to solve.
O 57% and 43%
O 50% and 50%
O 80 % and 20%
O 40% and 60%
Question 23
What is the atomic symbol for an element that has 30 neutrons and a mass
number of 55?
O Mn
O At
0 0 0
O Cs
O Hg
O Zn
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning