QUESTION 23 What is the sum of the coefficients (including implied 1's) after balancing the chemical reaction CaH1404(1) _O2lg) + co2te)_H20le) For example, the sum of the coefficients for 2 Hala) Oztg) 2 H20(gl would be 2+1+2 5, 1o the answer would be written an S QUESTION 24 Draw the best resonance structures for acetate. Which of the following is/are true? Select all that apply O Focusing on one resonance structure, there is one double bond and one single bond between the carbon and'oxygen atoms O The actual ion has two identical bonds between the carbon and oxygen atorms that are somowhere betwoen a double and triple bond O Focusing on one resonance structure, there is a 0 formal charge on one oxygen atom and a-1 formal charge on the other oxygen atom O Thore are three equally good resonance structures QUESTION 25 A 5.14 g sample of gas occupios 3.63 Lat a pressure of 878 torr and 22.58 °c. Calculate the molar mass of the gas in gimol Give your answer to 3 significant figures 760 torr= 1 atm
QUESTION 23 What is the sum of the coefficients (including implied 1's) after balancing the chemical reaction CaH1404(1) _O2lg) + co2te)_H20le) For example, the sum of the coefficients for 2 Hala) Oztg) 2 H20(gl would be 2+1+2 5, 1o the answer would be written an S QUESTION 24 Draw the best resonance structures for acetate. Which of the following is/are true? Select all that apply O Focusing on one resonance structure, there is one double bond and one single bond between the carbon and'oxygen atoms O The actual ion has two identical bonds between the carbon and oxygen atorms that are somowhere betwoen a double and triple bond O Focusing on one resonance structure, there is a 0 formal charge on one oxygen atom and a-1 formal charge on the other oxygen atom O Thore are three equally good resonance structures QUESTION 25 A 5.14 g sample of gas occupios 3.63 Lat a pressure of 878 torr and 22.58 °c. Calculate the molar mass of the gas in gimol Give your answer to 3 significant figures 760 torr= 1 atm
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter2: Lewis Structures
Section: Chapter Questions
Problem 11E: Below each structure in the previous question is a “condensed structure” that tells you...
Related questions
Question
3
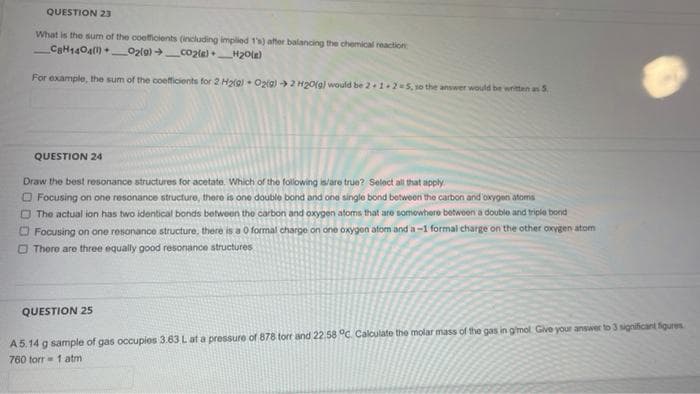
Transcribed Image Text:QUESTION 23
What is the sum of the coefficients (including implied 1's) after balancing the chemical reaction
CaH1404() _O2lg) co2te)_H20le)
For example, the sum of the coefficients for 2 Hala) Oztg) 2 H20(gl would be 2+1+2 5, so the answer would be written an S
QUESTION 24
Draw the best resonance structures for acetate. Which of the following islare true? Select all that apply
O Focusing on one resonance structure, there is one double bond and one single bond between the carbon and'oxygen atons
O The actual ion has two identical bonds betwoen the carbon and oxygen atoms that are somowhere betwoen a double and triple bond
O Focusing on one resonance structure, there is a 0 formal charge on one oxygen atom and a-1 formal charge on the other oxygen atom
O There are three equally good resonance structures
QUESTION 25
A5.14 g sample of gas occupios 3.63 Lat a pressure of 878 torr and 22.58 °c. Calculate the molar mass of the gas in gmol Give your answer to 3 significant figures
760 torr = 1 atm
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning