Separations: You have carried out the below reaction between an acid chloride with an amine to form an amide. Construct a flow chart to show how you would isolate the product from unreacted starting materials using a reactive acid base extraction. Keep in mind that acid chlorides are readily hydrolyzed to carboxylic acids in the presence of aqueous acid or base, and that amides are not extracted into aqueous acid or base. Assume you have DCM, 1.0M HCl, 1.0M NaOH, and saturated NaHCO. CI + H₂N excess CH2Cl2
Separations: You have carried out the below reaction between an acid chloride with an amine to form an amide. Construct a flow chart to show how you would isolate the product from unreacted starting materials using a reactive acid base extraction. Keep in mind that acid chlorides are readily hydrolyzed to carboxylic acids in the presence of aqueous acid or base, and that amides are not extracted into aqueous acid or base. Assume you have DCM, 1.0M HCl, 1.0M NaOH, and saturated NaHCO. CI + H₂N excess CH2Cl2
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter17: Carboxylic Acids
Section: Chapter Questions
Problem 17.53P
Related questions
Question
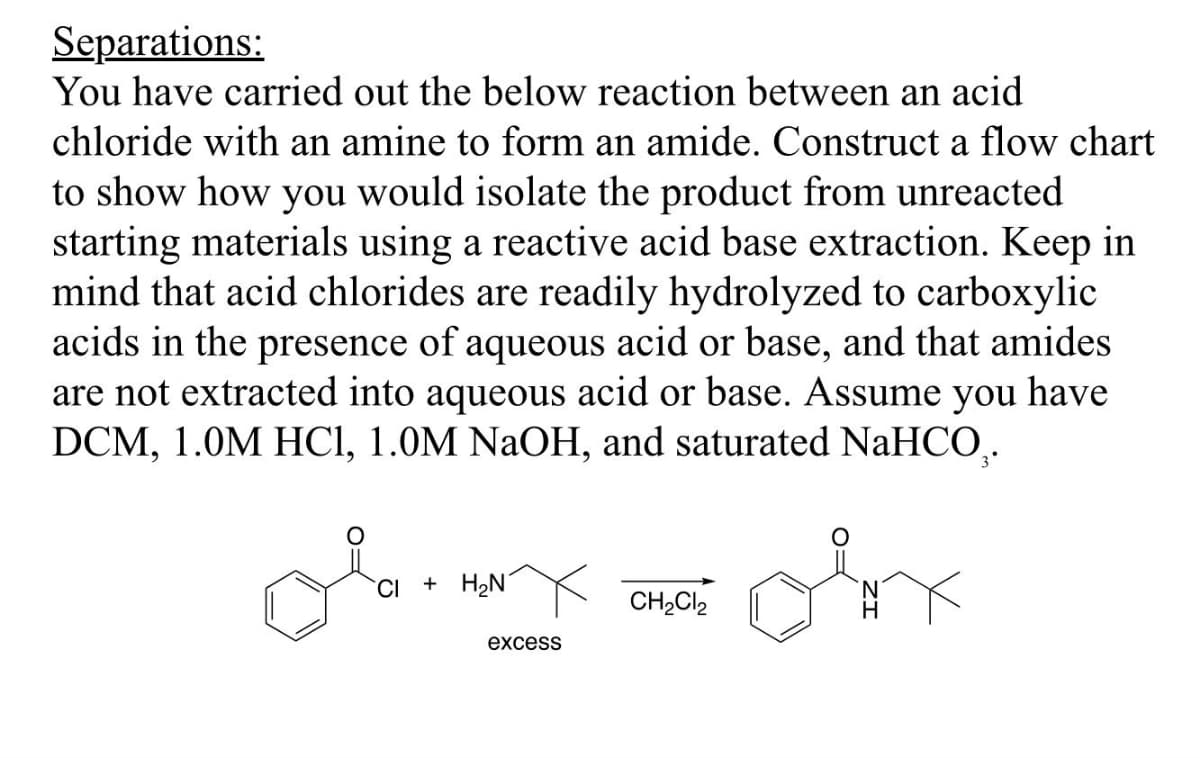
Transcribed Image Text:Separations:
You have carried out the below reaction between an acid
chloride with an amine to form an amide. Construct a flow chart
to show how you would isolate the product from unreacted
starting materials using a reactive acid base extraction. Keep in
mind that acid chlorides are readily hydrolyzed to carboxylic
acids in the presence of aqueous acid or base, and that amides
are not extracted into aqueous acid or base. Assume you have
DCM, 1.0M HCl, 1.0M NaOH, and saturated NaHCO.
CI + H₂N
excess
CH2Cl2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 3 images

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning