Starting from the internal energy equation, using the relations for enthalpy h = e + p/p and hT = h + u for the total enthalpy, get the following energy equation. Dhr Dt P- = p др д + div(kVT) + ox, -(Tij'ui) + pfiui
Q: Calculate the moment (positive if counterclockwise, negative if clockwise) of the 180-N force on the…
A: Moment of a force is the cross product of position vector and force. In given question we have to…
Q: Problem 10 The current density in a conducting medium is given by J = (zê-4y²y + 2x2) cos(wt) Find…
A: Current density J→ and charge density ρ are related by the continuity equation, which is written as…
Q: If city is located in 2.8° north latitude and 46.0° east longitude. From there, you want to fly to a…
A: To calculate the arc length between two points on a sphere, you can use the spherical law of…
Q: Through what potential difference would an electron need to be accelerated from rest to reach 40% of…
A:
Q: We have claimed that the following equation for charge on the capacitor of a series RLC circuit:…
A:
Q: A mass of 0.25 kg of air in a closed system expands from 2 bar and 6 0°C to 1 bar and 40° C, while…
A: Mass of air,m=0.25 kg Initial pressure,P1=2 bar=0.2×106N/m2Final pressure,P2=1…
Q: At double the distance from a long current-carrying wire, the strength of the magnetic field…
A:
Q: Please analyze the absolute error and relative error of the following three physical formulas: (1)…
A:
Q: Figure (a) shows the basic structure of a camera. A lens can be moved forward or back to produce an…
A: In Figure (a):- The distance between the lens and the film (lens-film distance) is given as f = 6.44…
Q: Using Area-Moment Method. Consider 150 mm x 150 mm section and E = 80 GPa. a=2.8 b=24.0…
A: To determine the vertical reaction at B and the moment reaction at A using the Area-Moment Method,…
Q: 7. Consider a circuit shown in figure switch was open for a long time and closed at t = 0. All…
A:
Q: Two long parallel wires carry currents of 5.0 A and 8.0 A in the opposite directions. The wires are…
A:
Q: Which of the followings is the current on the resistor R₂ as a function of time 1₂(t) after the…
A: Given Circuit diagram with compents resistors , capacitors and battery with potential V. Our task is…
Q: An arch of radius r = R has the charge of Q between ≤ ≤of nonuniform linear charge Ꮎ density given…
A: Given A linear charge system with charge Q and linear charge density is a function of angle , λ=…
Q: 2. For the given circuit, R. = 100 92, R₂ = R₁ = 50 92, and R4 25 2 a) Find the equivalent…
A: The resistances are given as follows R1=100Ω R2=R3=50Ω and R4=25Ω Lastly, the voltage source is V=12…
Q: A projectile is fired at an angle of 60.0° above the horizontal with an initial speed of 30.0 m/s.…
A:
Q: As a follow-up to the previous problem, the Morse oscillator for a different diatomic molecule has D…
A: The energy of the nth vibrational state of a Morse oscillator is given by: En = ħω [n + 12] - ħωx(n…
Q: 400 km altitude with flight path angle -10.0 degrees and speed 10.8 km/sec. a. Using Kepler's…
A: Given that:Flight path angle (γ) = -10.0 degreesSpeed at burnout (V) = 10.8 km/secRadius of the…
Q: explain by means of a one-line diagram: A) Reverse current protection method B) Method of…
A: Reverse current :When there is higher voltage at output of a system than at the input, causing…
Q: a) A uniform electric field has a magnitude of 2.3 x10³ In a vacuum, a proton begins with a speed of…
A:
Q: A.What is the potential difference between the plates. B. If the charge is kept constant, what will…
A:
Q: H1. A particle 1 of mass m₁ is attached to one end of a spring with constant k at position x = 0. It…
A: Mass of first particle=m1Speed of first particle, u1=0Mass of second particle=m2Speed of first…
Q: The ac generator in the figure supplies 120 V at 60.0 Hz. With the switch open as in the diagram,…
A:
Q: Activity 1: The motion equation of damped mass-spring system as shown in Fig. 1 below is given as…
A:
Q: 6-7. In Figure P6.7, the magnetic field is uniform and directed along the +y-direction. The…
A:
Q: A physics student is studying the effect of temperature on the resistance of a current carrying…
A:
Q: 7-3. In Figure PS-7-3, find the magnetic field at point P, which is at the common center of the two…
A: The magnetic field at a point P due to a current-carrying wire of very small length is given by the…
Q: 4. Consider a simple cubic system with lattice constant a = 5 Å. Determine the atom density…
A: A simple cubic lattice, the lattice constant 'a' is the distance between adjacent lattice points.…
Q: The Balmer series, or Balmer lines in atomic physics, is one of a set of six named series describing…
A: In Balmer series, Electrons transition to the energy level with n=2. We have to find the three…
Q: *[-]³ uniform line charge of length L and total charge +Q given in the figure. Electric field on the…
A: A rod having length L and charge Q is placed along y-axes. Then the magnitude charge density λ = Q/L…
Q: Two long parallel wires placed side-by-side on a horizontal table carry identical size currents in…
A:
Q: Consider a point particle of mass m moving in one dimension with potential V(x). The system is…
A:
Q: The spool has a mass of 30 kg and a radius of gyration of ko= 0.4 m, and r is 0.3 m. Neglect the…
A:
Q: s machine consists of blocks of masses m pulley without slipping. M T₁ || (a) Why must the tension…
A: Mass of block,m1 = 13 kg Mass of block,m2 = 17 kg Mass of solid cylinder,M = 6.50 Kg Radius of solid…
Q: In Fig 121, the conducting sphere A of radius R=20 cm that is initially isolated has has a potential…
A: Initially potential on sphere A is 10 V. And potential on sphere B is 0 because is it grounded.…
Q: 2. A new, exotic particle (not necessarily a Standard Model particle), X, is discovered which decays…
A: Based on the given decay mode of particle X, which is X → π^−μ^+, we can analyze the properties of X…
Q: Question 1. Find the steady state solution of the forced Mass-Spring-Damper with the following…
A:
Q: For a spherical mirror situation, you have determined that s = 12 cm and s' = 93 cm. What is the…
A: For the given question, the given mirror which is spherical, the object distance is u = -12cm and…
Q: Anna has a far point of infinity and near point of 80 cm, so Anna is Ofarsighted, should wear…
A: Nearsightedness and farsightedness are two common vision problems that affect people of all ages…
Q: Find the potential on the z axis (0,0,h) produced by an annular(s) ring of uniform surface charge…
A: Region occupied by the ring is given by z=0 , ρ≤ a, 0≤ φ≤2π
Q: A wave is described by the following wave function, y(x, t) = 0,06 sin (0,02mx-4rt) If the wave…
A: Power transported by a wave depends upon mass per unit length, amplitude of the wave, angular…
Q: Based on the shell model assign spin and parity to the ground states of 32S, 3K and Ni. 16
A:
Q: a) Calculate the excitation energies for the 1s→ 3p electron transition for the H-atom and for the…
A: Energy of electron in Hydrogen like atom depends upon principal quantum number of the orbit.
Q: Questions 13, 14 and 15 refer to the diagram below: 0, 14. *A. B. C. D. T₁ 13. Which of the…
A: Tension is the force exerted by a cable or rope, or similar object. When a rope or cable is hung or…
Q: 1. Am = 0.50 kg cart attached to a spring is pulled a distance D = 0.20 m to the right from the…
A: The cart of mass 0.5 kg is attached to the spring, and is pulled to a distance of 0.2 m to the right…
Q: 19) The resistance of R, is 50 and R, is 100. R, is made of a nichrome wire (resistivity of 1.5 x 10…
A: The resistance R and the resistivity ρ of a wire are related to each other by the formula, R=ρLA…
Q: Red light of vacuum wavelength 650 nm is travelling in non-magnetic glass with refractive index nG =…
A:
Q: Sam is nearsighted with a far point of 185 cm. What refractive power should be prescribed fo Sam to…
A: Given Data: far point = 185cm = 1.85m to correct vision we need to shift far point at infinity To…
Q: 1. Alice and Bob prepare two qubits in the state [4), |4) = 3/7|00) + 6/7|01) + 2/7/10) (a)…
A: It is given that |¥) = 3/7|00) + 6/7|01) + 2/7|10) Determine the concurrence of state and…
Q: A solenoid carries a current I. An electron is injected with velocity v along the axis AB of the…
A: If the electron has velocityv→, magnetic field along axis is B→ and particle has charge q then…
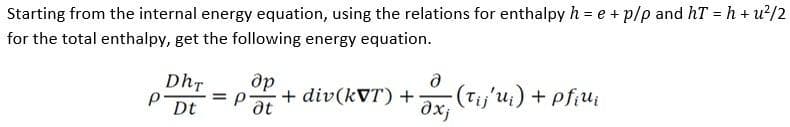
Step by step
Solved in 4 steps
