System A and system B are thermally isolated from everything else but exchange heat with each other. System A is initially at 300 K and system B is initially at 400 K. System A has mass 2.0 kg and specific heat 1000 J/kg K. System B has mass 1.0 kg and the same specific heat of 1000 J/ kg K. What is the net (total) change in entropy from the initial state to the final equilibrium state? Hint: you need to determine the final equilibrium temperature. B
System A and system B are thermally isolated from everything else but exchange heat with each other. System A is initially at 300 K and system B is initially at 400 K. System A has mass 2.0 kg and specific heat 1000 J/kg K. System B has mass 1.0 kg and the same specific heat of 1000 J/ kg K. What is the net (total) change in entropy from the initial state to the final equilibrium state? Hint: you need to determine the final equilibrium temperature. B
Related questions
Question
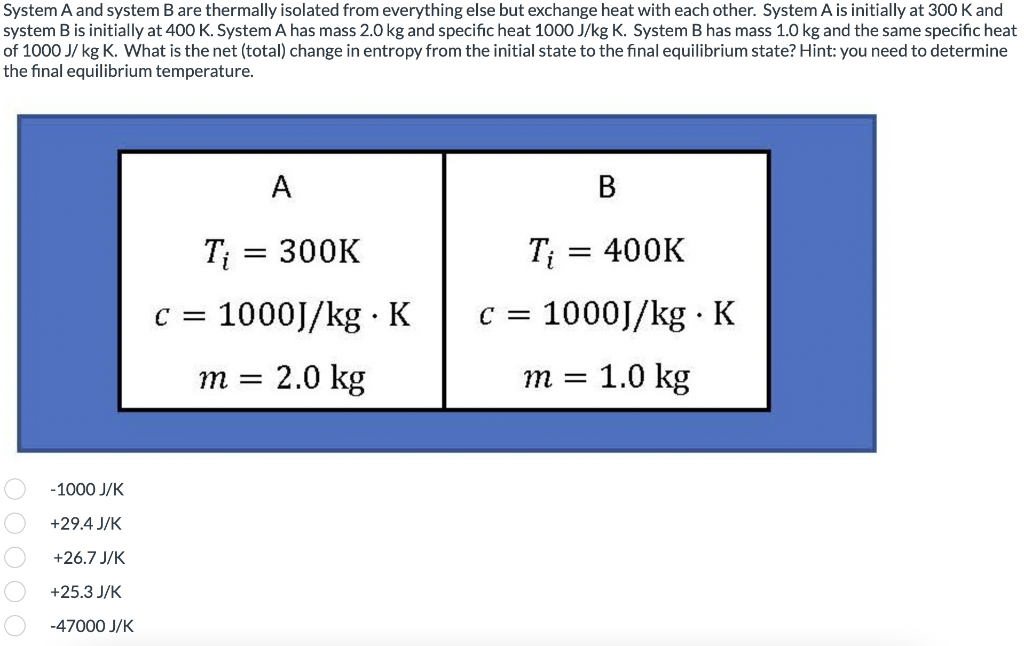
Transcribed Image Text:System A and system B are thermally isolated from everything else but exchange heat with each other. System A is initially at 300 K and
system B is initially at 400 K. System A has mass 2.0 kg and specific heat 1000 J/kg K. System B has mass 1.0 kg and the same specific heat
of 1000 J/ kg K. What is the net (total) change in entropy from the initial state to the final equilibrium state? Hint: you need to determine
the final equilibrium temperature.
-1000 J/K
+29.4 J/K
+26.7 J/K
+25.3 J/K
-47000 J/K
A
T₁ = 300K
C = 1000J/kg. K
m = 2.0 kg
B
Ti = 400K
C = 1000J/kg. K
1.0 kg
m =
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps
