Table 19.3 Elution sequence for compounds Hydrocarbons Fastest (will elute with nonpolar solvent) Olefins Ethers Halocarbons Aromatics Ketones Order of elution Aldehydes Esters Alcohols Amines Acids, strong bases Slowest (need a polar solvent)
Table 19.3 Elution sequence for compounds Hydrocarbons Fastest (will elute with nonpolar solvent) Olefins Ethers Halocarbons Aromatics Ketones Order of elution Aldehydes Esters Alcohols Amines Acids, strong bases Slowest (need a polar solvent)
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter22: Biochemistry
Section: Chapter Questions
Problem 68E
Related questions
Question
Consider a sample that is a mixture composed of biphenyl, benzoic acid, and benzyl alcohol. The sample is spotted on a TLC plate and developed in a dichloromethane– cyclohexane solvent mixture. Predict the relative Rf values for the three components in the sample. Hint: See Table .
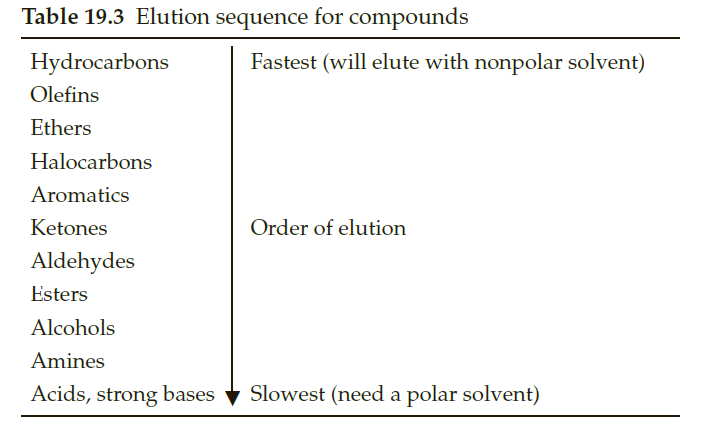
Transcribed Image Text:Table 19.3 Elution sequence for compounds
Hydrocarbons
Fastest (will elute with nonpolar solvent)
Olefins
Ethers
Halocarbons
Aromatics
Ketones
Order of elution
Aldehydes
Esters
Alcohols
Amines
Acids, strong bases
Slowest (need a polar solvent)
Expert Solution
Step 1
Given data,
Consider a sample that is a mixture composed of biphenyl, benzoic acid, and benzyl alcohol. The sample is spotted on a TLC plate and developed in a dichloromethane-cyclohexane solvent mixture.
The relative Rf values for the three components in the sample have to be predicted.
The formula for the calculating relative Rf value has to be given below,
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning