The extraction of aluminum metal from the aluminum hydroxide in bauxite ore by the Hall-Héroult process is one of the most remarkable success stories of late 19th century practical chemistry, turning aluminum from a rare and precious metal into the cheap commodity it is today. In the first step, aluminum hydroxide reacts to form alumina and water: 2 Al(OH),(s)→ Al,03(s)+3 H,0(g) In the second step, alumina and carbon react to form aluminum and carbon dioxide: 2 Al,0,(s)+3 C(s)→ 4 Al(s)+3 CO,(g) Write the net chemical equation for the production of aluminum from aluminum hydroxide and carbon. Be sure your equation is balanced. ロ→ロ
The extraction of aluminum metal from the aluminum hydroxide in bauxite ore by the Hall-Héroult process is one of the most remarkable success stories of late 19th century practical chemistry, turning aluminum from a rare and precious metal into the cheap commodity it is today. In the first step, aluminum hydroxide reacts to form alumina and water: 2 Al(OH),(s)→ Al,03(s)+3 H,0(g) In the second step, alumina and carbon react to form aluminum and carbon dioxide: 2 Al,0,(s)+3 C(s)→ 4 Al(s)+3 CO,(g) Write the net chemical equation for the production of aluminum from aluminum hydroxide and carbon. Be sure your equation is balanced. ロ→ロ
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter5: Gases
Section: Chapter Questions
Problem 5.62PAE: 62 Ammonium dinitramide (ADN), NH4N(NO2)2, was considered as a possible replacement for aluminium...
Related questions
Question
100%
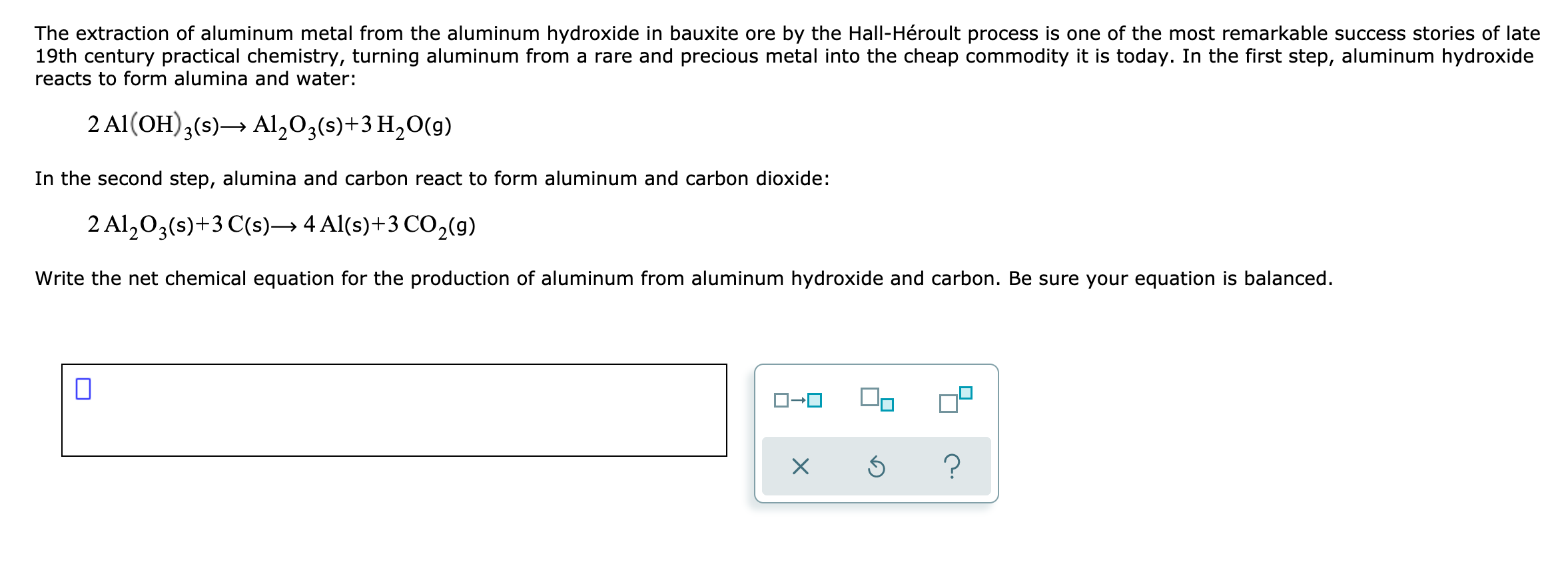
Transcribed Image Text:The extraction of aluminum metal from the aluminum hydroxide in bauxite ore by the Hall-Héroult process is one of the most remarkable success stories of late
19th century practical chemistry, turning aluminum from a rare and precious metal into the cheap commodity it is today. In the first step, aluminum hydroxide
reacts to form alumina and water:
2 Al(OH),(s)→ Al,03(s)+3 H,0(g)
In the second step, alumina and carbon react to form aluminum and carbon dioxide:
2 Al,0,(s)+3 C(s)→ 4 Al(s)+3 CO,(g)
Write the net chemical equation for the production of aluminum from aluminum hydroxide and carbon. Be sure your equation is balanced.
ロ→ロ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning