Which one of the following statements is not true? In mass spectrometry, the initially formed radical cation can sometimes survive and appear as a peak on the mass spe Visible light is higher in energy than infrared light. O In mass spectrometry the peak corresponding to an ethyl cation fragment would appear at m/z 30. In mass spectrometry, a benzylic cation would appear at m/z 91. O In IR spectroscopy, a C-O bond stretch would appear at a lower cm than a C-O bond stretch. n-1
Which one of the following statements is not true? In mass spectrometry, the initially formed radical cation can sometimes survive and appear as a peak on the mass spe Visible light is higher in energy than infrared light. O In mass spectrometry the peak corresponding to an ethyl cation fragment would appear at m/z 30. In mass spectrometry, a benzylic cation would appear at m/z 91. O In IR spectroscopy, a C-O bond stretch would appear at a lower cm than a C-O bond stretch. n-1
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
ChapterL2: Mass Spectrometry
Section: Chapter Questions
Problem 3E
Related questions
Question
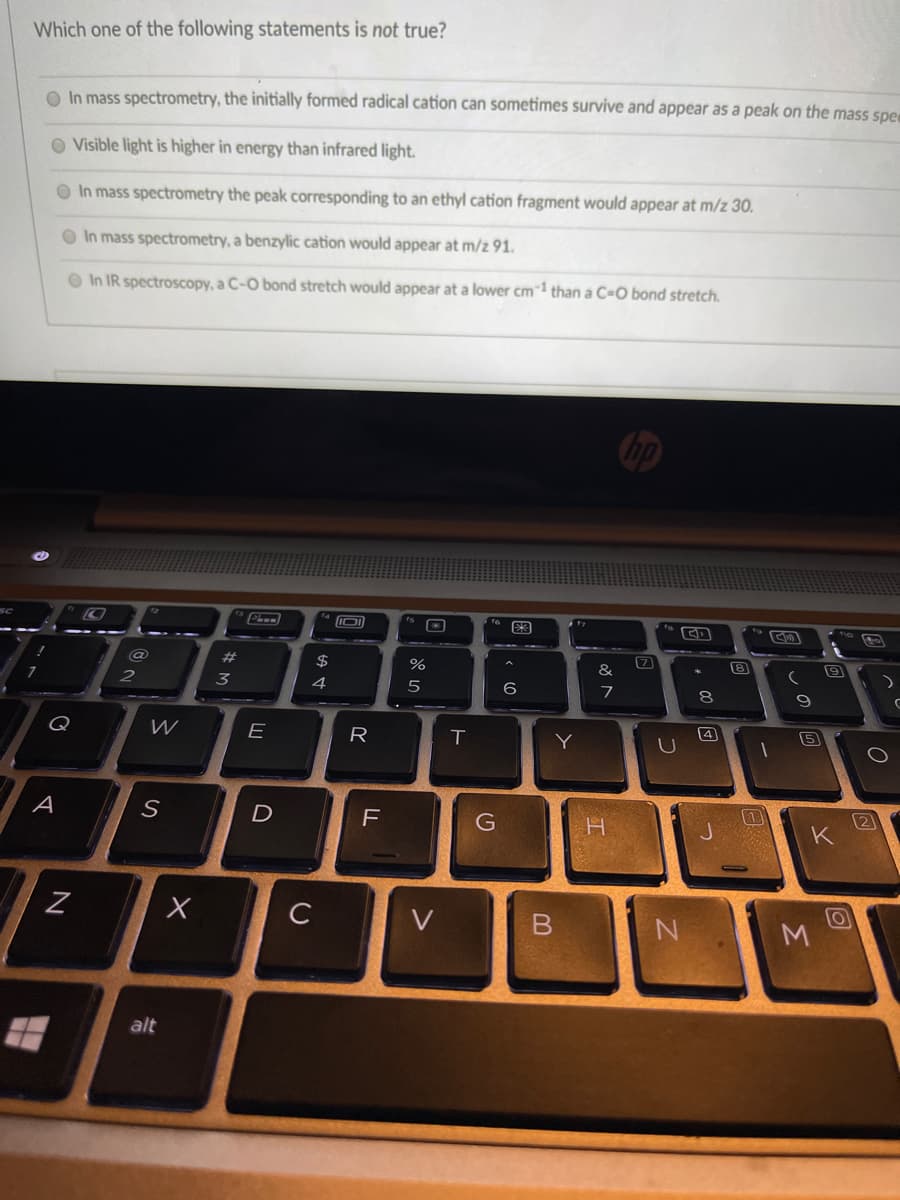
Transcribed Image Text:Which one of the following statements is not true?
O In mass spectrometry, the initially formed radical cation can sometimes survive and appear as a peak on the mass spe
O Visible light is higher in energy than infrared light.
O In mass spectrometry the peak corresponding to an ethyl cation fragment would appear at m/z 30.
O In mass spectrometry, a benzylic cation would appear at m/z 91.
O In IR spectroscopy, a C-O bond stretch would appear at a lower cm1 than a C=O bond stretch.
%23
$
&
8
2
3
4
6
8
Q
E
R
T.
Y
4
5
2
K
D
G
H.
C
|| N
M.
alt
Expert Solution
Step 1
Spectroscopy is a technique using which the structure of a chemical compound is analyzed and bonding between atoms present is determined. Infrared spectroscopy, 1H and 13C NMR spectroscopy, and mass spectroscopy are some of the most commonly used spectroscopy techniques.
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning