You are given four values (1.033, 4.157, 1.05, and 4.6) and asked to conduct calculations on them. You perform a series of mathematical operations using the values and make a table that includes the calculated value as displayed on your calculator: Mathematical operation Value displayed by calculator 1.033 + 4.157 +1.05 + 4.6 10.8400 1.033 x 1.05 1.08465 4.157 1.05 3.10700 4.157/31.0341 0.133949 4.6/31.0341 0.148224 Round each of the values displayed by the calculator to the correct number of significant figures, and place the value in the bin that correctly indicates the number of significant digits you rounded to. Drag the appropriate items o their respective bins. • View Available Hint(s) Reset Help 10.8400 1.084653.10700 0.133949 0.148224 1 significant figure 2 significant figures 3 significant figures 4 significant figures
You are given four values (1.033, 4.157, 1.05, and 4.6) and asked to conduct calculations on them. You perform a series of mathematical operations using the values and make a table that includes the calculated value as displayed on your calculator: Mathematical operation Value displayed by calculator 1.033 + 4.157 +1.05 + 4.6 10.8400 1.033 x 1.05 1.08465 4.157 1.05 3.10700 4.157/31.0341 0.133949 4.6/31.0341 0.148224 Round each of the values displayed by the calculator to the correct number of significant figures, and place the value in the bin that correctly indicates the number of significant digits you rounded to. Drag the appropriate items o their respective bins. • View Available Hint(s) Reset Help 10.8400 1.084653.10700 0.133949 0.148224 1 significant figure 2 significant figures 3 significant figures 4 significant figures
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1ALQ: a. There are 365 days per year, 24 hours per day, 12 months per year, and 60 minutes per hour. Use...
Related questions
Question
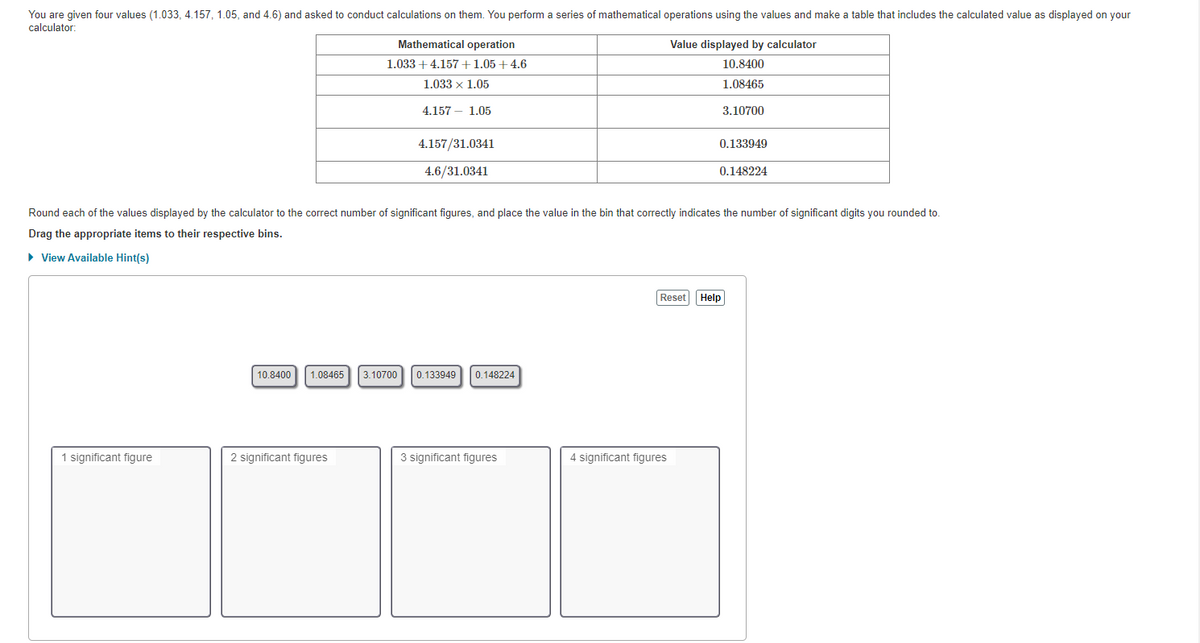
Transcribed Image Text:You are given four values (1.033, 4.157, 1.05, and 4.6) and asked to conduct calculations on them. You perform a series of mathematical operations using the values and make a table that includes the calculated value as displayed on your
calculator:
Mathematical operation
Value displayed by calculator
1.033 +4.157 +1.05+4.6
10.8400
1.033 x 1.05
1.08465
4.157 - 1.05
3.10700
4.157/31.0341
0.133949
4.6/31.0341
0.148224
Round each of the values displayed by the calculator to the correct number of significant figures, and place the value in the bin that correctly indicates the number of significant digits you rounded to.
Drag the appropriate items to their respective bins.
» View Available Hint(s)
Reset Help
10.8400
1.08465
3.10700
0.133949
0.148224
1 significant figure
2 significant figures
3 significant figures
4 significant figures
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning