
Concept explainers
Draw a Lewis structure for each ion.
a.
(a)
Interpretation: The Lewis structure for
Concept Introduction: Lewis structures are electron dot representations for molecules. These structures show the bonding between the atoms or molecules and the lone pairs of electrons. The arrangement of valence electrons among the atoms in a molecule is represented by its Lewis structure.
Answer to Problem 1.7P
The Lewis structure for
Explanation of Solution
In the given molecular formula,
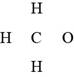
Figure 1
The total valence electrons in
The bonds and lone pairs are added to form
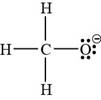
Figure 2
The Lewis structure for
(b)
Interpretation: The Lewis structure for
Concept Introduction: Lewis structures are electron dot representations for molecules. These structures show the bonding between the atoms or molecules and the lone pairs of electrons. The arrangement of valence electrons among the atoms in a molecule is represented by its Lewis structure.
Answer to Problem 1.7P
The Lewis structure of
Explanation of Solution
In the given molecular formula,
![]()
Figure 3
The total valence electrons in
The bonds and lone pairs are added to form
![]()
Figure 4
The Lewis structure for
![]()
Figure 5
The Lewis structure of
(c)
Interpretation: The Lewis structure for
Concept Introduction: Lewis structures are electron dot representations for molecules. These structures show the bonding between the atoms or molecules and the lone pairs of electrons. The arrangement of valence electrons among the atoms in a molecule is represented by its Lewis structure.
Answer to Problem 1.7P
The Lewis structure of
Explanation of Solution
In the given molecular formula,
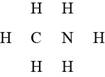
Figure 6
The total valence electrons in
The bonds and lone pairs are added to form
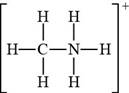
Figure 7
The Lewis structure of
(d)
Interpretation: The Lewis structure for
Concept Introduction: Lewis structures are electron dot representations for molecules. These structures show the bonding between the atoms or molecules and the lone pairs of electrons. These structures can be drawn for any covalently bonded molecules.
Answer to Problem 1.7P
The Lewis structure of
Explanation of Solution
The given molecular formula,
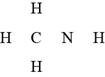
Figure 8
The total valence electrons in
The bonds and lone pairs are added to form
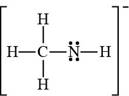
Figure 9
The Lewis structure of
Want to see more full solutions like this?
Chapter 1 Solutions
Organic Chemistry
Additional Science Textbook Solutions
Chemistry In Context
Chemistry: Matter and Change
Chemistry (7th Edition)
Living By Chemistry: First Edition Textbook
Organic Chemistry As a Second Language: Second Semester Topics
- Draw the Lewis structure for CH4O. b. Draw the Lewis structure for HNO2arrow_forwardDraw the attraction between a water molecule and a molecule of BrCl4F. Br is the central atom in an expanded octet - count your electrons carefully.arrow_forwardDraw the lewis structure and use curved arrows to show the movement of electrons. CaC2 + 2H2O → Ca(OH)2 + C₂H₂arrow_forward
- 1. Why do you think we avoid writing the minus sign (-) in chemical equations? 2. What does CO3?- have to do with ocean acidification? Explain the chemical reactions completely. 3. One of the harmful oxidants in the body is the neutral OH• molecule. It has 7 total valence electrons, and unlike the OH- ion, it has no charge. Draw the Lewis structure of a regular OH- ion, then draw the Lewis structure of OH• and use this to show why OH• has very high energy, and a high tendency to oxidize the biomolecules inside our cells. (Recall what you learned from modules 2 and 3.) 4. If I weigh 40 kg, what is my molar mass? In other words, if we made trillions and trillions of my clones to make 1 mole of me, what would be our mass all together? Show your solution with units. 5. When coal is burned, sulfur impurities in the coal react with oxygen gas to form sulfur trioxide: S+ 02 → S03 a. Did oxidation occur? Explain. b. Balance the equation. c. Translate the equation into a sentence by filling in…arrow_forwardFormula Lewis Electron Molecular Bond Polar or Attractive Structure Pair Geometry Angle Force Non Polar Geometry Between Molecules CH4 Around First C Around First C C2H4 Around First C Around First C C,H2 Around First C Around First C CH;OH Around O Around O C2H;OH Around O Around O CH20 CH,OCH, Around O Around O CH;COOH Central C Central C НСООН Central C Central C CH;NH2 Around N Around N CH;COCH; Central C Central Carrow_forwardDraw the Lewis structure for each organic compound from its condensed structural formula. a. CH4 b. CH,NH2 с. НСНО d. CH,CH2OH е. НСООНarrow_forward
- 25.Which of the following does have H- bond? electronegativities: ) = 3.5, H = 2.1, C = 2.5, Cl = 3.0, S = 2.8 O || I. H- C- O-H II. SCl2 III . H | H - C - S -H | H IV. H H - C - O - H | H a.IIIb.IIc.I, II, IVd.I and IVarrow_forwardWrite Lewis structures for each molecule or ion. Include reso- nance structures if necessary and assign formal charges to all atoms. If necessary, expand the octet on the central atom to lower formal charge. a. SO,2- b. HSO, c. SO3 d. BrOzarrow_forwardConsider the following ion: BrO3¯. a) Show the full electron configuration for Br. b) Draw the most correct Lewis structure for BrO3¯ and briefly explain why your Lewis structure is correct. c) If the structure is stabilised by resonance, draw at least one of the possible resonance forms. If it is not stabilised by resonance, briefly explain why. d) What is the electronic geometry of BrO3-? What is its molecular shape? e) Does BrO3 have a dipole moment? Briefly justify your answer. f) On average, would you expect IO3¯ to have longer or shorter bonds than BrO3¯? Briefly explain your answer. g) Which of the following molecules would you expect to have the lowest vapour pressure? Briefly explain your choice. Br HO HO. Br- Compound A Compound B Compound C h) What is the molecular formula for Compound C? What is the empirical formula for Compound C?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





