
Concept explainers
(a)
Interpretation:
The dipole moment arrow is to be drawn for the given C-F bond.
Concept introduction:
Dipole moment arrows are drawn from the atom with less electronegativity to atom with more electronegativity.
(a)
Answer to Problem 17A
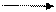
Explanation of Solution
Fluorine is more electronegative than carbon because left to right electronegativity increases. So, arrow will be from carbon to fluorine.
(b)
Interpretation:
The dipole moment arrow is to be drawn for the given Si-C bond.
Concept introduction:
Dipole moment arrows are drawn from the atom with less electronegativity to atom with more electronegativity.
(b)
Answer to Problem 17A
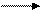
Explanation of Solution
Carbon is more electronegative than silicon because down the group electronegativity decreases. So, arrow will be from carbon to silicon.
(c)
Interpretation:
The dipole moment arrow is to be drawn for the C-O bond.
Concept introduction:
Dipole moment arrows are drawn from the atom with less electronegativity to atom with more electronegativity.
(c)
Answer to Problem 17A
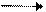
Explanation of Solution
Oxygen is more electronegative than carbon because left to right electronegativity increases. So, arrow will be from carbon to oxygen.
(d)
Interpretation:
The dipole moment arrow is to be drawn for the given B-C bond.
Concept introduction:
Dipole moment arrows are drawn from the atom with less electronegativity to atom with more electronegativity.
(d)
Answer to Problem 17A

Explanation of Solution
Carbon is more electronegative than boron because left to right electronegativity increases. So, arrow will be from carbon to boron.
Chapter 12 Solutions
World of Chemistry, 3rd edition
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





