
Concept explainers
(a)
Interpretation:
The Lewis structure of given molecule is to be drawn.
Concept introduction:
Lewis structure is a representation of a molecule which shows shared and unshared pair of electrons. It is helpful to determine the shape of a molecule.
(a)
Answer to Problem 64A
Structure of
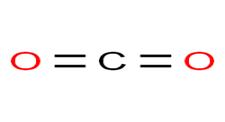
Structure of
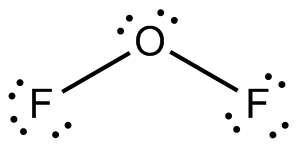
Explanation of Solution
In
In
(b)
Interpretation: The molecular geometry of given molecules is to be written.
Concept introduction:
Structure of molecule can be drawn using VSEPR theory. The total number of bonding and non-bonding pairs of electrons tells about the geometry of the molecule.
(b)
Answer to Problem 64A
Geometry of
Geometry of
Explanation of Solution
In
In
(c)
Interpretation: The molecular geometry of given molecules is to be written.
Concept introduction:
Structure of molecule can be drawn using VSEPR theory. The total number of bonding and non-bonding pairs of electrons tells about the geometry and molecular structure of the molecule.
(c)
Answer to Problem 64A
Structure (shape) of
Structure (shape) of
Explanation of Solution
In
In
(d)
Interpretation:
The shape of given molecules is to be compared.
Concept introduction:
Two molecules having same general formula can sometimes have same shapes and sometimes have different shapes. It depends on type of pair of valence electrons around the central atom.
(d)
Answer to Problem 64A
Explanation of Solution
In
In
Chapter 12 Solutions
World of Chemistry, 3rd edition
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





