
Concept explainers
Interpretation:
The difference between a bonding pair and a lone pair of electrons is to be written.
Concept introduction:
Electrons are the subatomic particles which are used in bonding of atoms.
During bond formation in covalent bonding, some electrons are shared and some remains unshared.
Explanation of Solution
In a covalent bond, the electron pair which is shared between the two atoms is bonding pair of electrons and the pair of electrons left unshared on atom is called as lone pair of electrons.
Let’s study the difference using example:
Lewis dot structure of
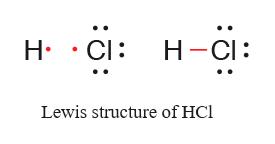
It shows one electron pair is shared between both atoms. That pair of electrons is bonding pair of electrons.
There are three electron pairs present on chlorine which are not involved in bond formation are lone pairs of electrons.
Thus, pair of electrons involved in bond formation are bonding and which are not involved in bond formation are lone pairs of electrons.
Chapter 12 Solutions
World of Chemistry, 3rd edition
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





