
Concept explainers
(a)
Interpretation: The Lewis and resonating structures of given compound PCl3is to be written and valence electrons are to be counted.
Concept introduction: Lewis structure shows sharing of electrons using dots. Each
(a)
Answer to Problem 4RQ
Sum of valence electrons of all atoms=26
Lewis structure with circles:
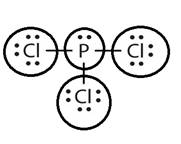
Explanation of Solution
Sum of valence electrons of all atoms=5+7+7+7=26
Lewis structure with circles:
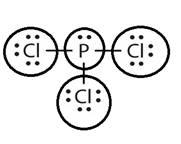
Phosphorus has five electrons and chlorine has seven electrons in valence shell. Thus, each chlorine atom shares one electron with central phosphorus atom to complete the octet.
From Lewis structure, octet of phosphorus and chlorine is complete.
There is no resonance in it.
(b)
Interpretation: The Lewis and resonating structures of given compound Br2is to be written and valence electrons are to be counted.
Concept introduction: Lewis structure shows sharing of electrons using dots. Each atom has number of dots equal to valence electrons. Every atom shares electrons to complete eight electrons in valence shell. Resonating structures are drawn when one structure can’t explain all the properties of molecule.
(b)
Answer to Problem 4RQ
Sum of valence electrons of all atoms=14
Lewis structure with circles:
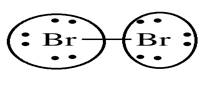
Explanation of Solution
Sum of valence electrons of all atoms=7+7=14
Lewis structure with circles:
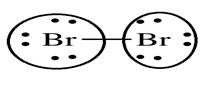
Bromine belongs to 17th group. So, it has seven electrons in its valence shell. It requires one electron to complete its octet thus share one electron with other bromine atom.
From Lewis structure, octet of bromine is complete.
There is no resonance in
(c)
Interpretation: The lewis and resonating structures of given compound
Concept introduction: Lewis structure shows sharing of electrons using dots. Each atom has number of dots equal to valence electrons. Every atom shares electrons to complete eight electrons in valence shell. Resonating structures are drawn when one structure can’t explain all the properties of molecule.
(c)
Answer to Problem 4RQ
Sum of valence electrons of all atoms=18
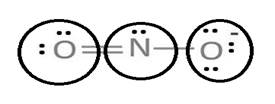
Lewis structure with circles:
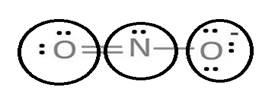
Resonating structure:
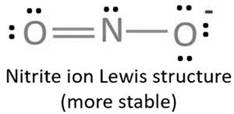
Explanation of Solution
Sum of valence electrons of all atoms=6+5+6+1=18
Lewis structure with circles:
Resonating structure:
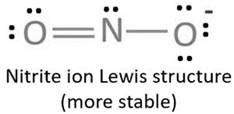
Lewis structure of nitrite ion shows nitrogen shares its one electron with oxygen atom carrying negative charge to complete its octet and two electrons with neutral oxygen atom to complete their octet.
But, experiment shows both the N-O bond lengths are equal. So, it has resonance in which negative charge is equally spread on both oxygen atoms and both N-O bond lengths lie in between single and double bond.
From Lewis structure, octet of both oxygen and nitrogen is complete.
(d)
Interpretation: The lewis and resonating structures of given compound O3 is to be written and valence electrons are to be counted.
Concept introduction: Lewis structure shows sharing of electrons using dots. Each atom has number of dots equal to valence electrons. Every atom shares electrons to complete eight electrons in valence shell. Resonating structures are drawn when one structure can’t explain all the properties of molecule.
(d)
Answer to Problem 4RQ
Sum of valence electrons of all atoms=18
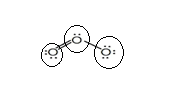
Lewis structure with circles:
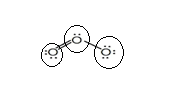
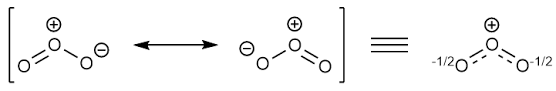
Resonating structure:
Explanation of Solution
Sum of valence electrons of all atoms=6+6+6=18
Lewis structure with circles:
Resonating structure:
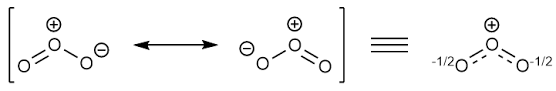
The formula of ozone is
From Lewis structure, octet of all oxygen is complete.
(e)
Interpretation: The lewis and resonating structures of given compound is to be written and valence electrons are to be counted.
Concept introduction: Lewis structure shows sharing of electrons using dots. Each atom has number of dots equal to valence electrons. Every atom shares electrons to complete eight electrons in valence shell. Resonating structures are drawn when one structure can’t explain all the properties of molecule.
(e)
Answer to Problem 4RQ
Sum of valence electrons of all atoms=10
Lewis structure with circles:
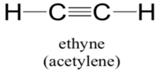
Explanation of Solution
Sum of valence electrons of all atoms=4+4+1+1=10
Lewis structure with circles:
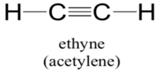
Carbon atom has four electrons in valence shell. It shares one electron with hydrogen atom to complete doublet of hydrogen. Now, to complete octet it shares three electrons with other carbon atom to complete octet and thus forms triple bond.
From Lewis structure, octet of all carbon is complete and doublet of hydrogen is complete.
(f)
Interpretation: The lewis and resonating structures of given compound is to be written and valence electrons are to be counted.
Concept introduction: Lewis structure shows sharing of electrons using dots. Each atom has number of dots equal to valence electrons. Every atom shares electrons to complete eight electrons in valence shell. Resonating structures are drawn when one structure can’t explain all the properties of molecule.
(f)
Answer to Problem 4RQ
Sum of valence electrons of all atoms=18
Lewis structure with circles:
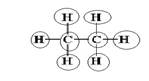
Explanation of Solution
Sum of valence electrons of all atoms=4+4+1+1+1+1+1+1=18
Lewis structure with circles:
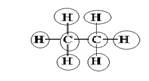
Carbon has four electrons in valence shell. So, each carbon forms one single bond with other carbon atom and three single bonds with three hydrogen atoms.
From Lewis structure, octet of all carbon is complete and doublet of hydrogen is complete.
(g)
Interpretation: The lewis and resonating structures of given compound is to be written and valence electrons are to be counted.
Concept introduction: Lewis structure shows sharing of electrons using dots. Each atom has number of dots equal to valence electrons. Every atom shares electrons to complete eight electrons in valence shell. Resonating structures are drawn when one structure can’t explain all the properties of molecule.
(g)
Answer to Problem 4RQ
Sum of valence electrons of all atoms=10
Lewis structure with circles:
Explanation of Solution
Sum of valence electrons of all atoms=1+4+5=10
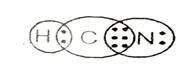
Lewis structure with circles:
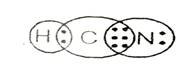
Lewis structure shows there is single bond between carbon and hydrogen and triple bond between carbon and nitrogen.
From Lewis structure, octet of nitrogen and carbon is complete and doublet of hydrogen is complete.
(h)
Interpretation: The lewis and resonating structures of given compound is to be written and valence electrons are to be counted.
Concept introduction: Lewis structure shows sharing of electrons using dots. Each atom has number of dots equal to valence electrons. Every atom shares electrons to complete eight electrons in valence shell. Resonating structures are drawn when one structure can’t explain all the properties of molecule.
(h)
Answer to Problem 4RQ
Sum of valence electrons of all atoms=23
Lewis structure with circles:
Resonating structure:
Explanation of Solution
Sum of valence electrons of all atoms=4+6+6+6+1=23
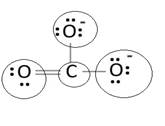
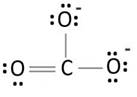
Lewis structure with circles:
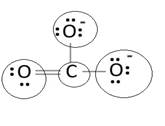
Resonating structure:
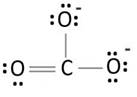
Lewis structure of carbonate ion shows one oxygen atom is bonded to carbon by double bond and two oxygen ions are bonded by single bonded. But in actual all the C-O bond lengths in carbonate ion is same. So, resonating structures of carbonate ions are formed. In resonance hybrid two unit negative charge is equally spread on three oxygen atoms and bond length of C-O lies in between double and single bond.
Lewis structure shows octet of oxygen and carbon is complete.
Chapter 12 Solutions
World of Chemistry, 3rd edition
- Each of the highlighted carbon atoms is connected to hydrogen atoms.arrow_forwardく Complete the reaction in the drawing area below by adding the major products to the right-hand side. If there won't be any products, because nothing will happen under these reaction conditions, check the box under the drawing area instead. Note: if the products contain one or more pairs of enantiomers, don't worry about drawing each enantiomer with dash and wedge bonds. Just draw one molecule to represent each pair of enantiomers, using line bonds at the chiral center. More... No reaction. Explanation Check O + G 1. Na O Me Click and drag to start drawing a structure. 2. H + 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility 000 Ar Parrow_forwardDraw a tetramer of this alternating copolymer.arrow_forward
- H I T H HH H -H C. H- Identify and select all structures below that represent a constitutional isomer(s) of the compound shown above. H- H CIH H H H HHHH H H 0 ·H H– 冊 CH CHI HH C- H- H H- H H A. H H C H H- -H HH H B. H- -H D. H H H H • H -H E. -H H H HICH T HHH F. H-arrow_forwardPolylactic acid (shown below) is a biodegradable polymer used for food packaging. Identify the monomer(s) used in the production of this polymer using a condensation process.arrow_forwardDraw the product of the reaction shown below. Ignore small byproducts that would evaporate pleasearrow_forward
- Poly(ethylene adipate) is a biodegradable polyester (shown below). Identify the type of polymerization process used in the production of this polymer.arrow_forwardPolymers may be composed of thousands of monomers. draw two repeat units(dimer) of the polymer formed in this reaction. assume there are hydrogen atoms on the two ends of the dimer. ignore inorganic byproducts pleasearrow_forwardDraw the product of the reaction shown below. Use a dash or wedge bond to indicate stereochemistry of substituents on asymmetric centers, Ignore inorganic byproductsarrow_forward
- Draw the product of this reaction please. Ignore inorganic byproductsarrow_forwardOne of the pi molecular orbitals of 1,3-butadiene (CH2=CHCH=CH2) is shown below. Please identify the number of nodal planes perpendicular to the bonding axisarrow_forwardDraw the monomers required to synthesize this condensation polymer please.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





