
Inorganic Chemistry
5th Edition
ISBN: 9780321811059
Author: Gary L. Miessler, Paul J. Fischer, Donald A. Tarr
Publisher: Prentice Hall
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4, Problem 4.28P
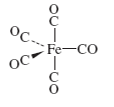
For the following molecules, determine the number of IR-active
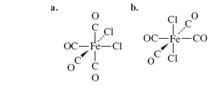
c.
Expert Solution & Answer
Learn your wayIncludes step-by-step video

schedule08:44
Students have asked these similar questions
Which of the following has the largest CN stretching energy?
OA.A
OB. B
OC.C
O D.D
Ο Ε.Ε
C
O
NH₂
IZ
A
O
D
N
B
EN
O
E
ZI
N
Why different molecular bonds have different IR spectroscopic peaks or bands?
What wavelength is the peak absorbance of CoCl2?
Chapter 4 Solutions
Inorganic Chemistry
Ch. 4.1 - Prob. 4.1ECh. 4.1 - Find all the symmetry elements in the following...Ch. 4.2 - Use the procedure described previously to verify...Ch. 4.3 - Prob. 4.4ECh. 4.3 - Verify the transformation matrices for the E and...Ch. 4.3 - Prepare a representation flowchart according to...Ch. 4.4 - Which point groups are possible for chiral...Ch. 4.4 - Write the corresponding 99 transformation matrices...Ch. 4.4 - Using the x, y, and z coordinates for each atom in...Ch. 4.4 - Reduce the following representations to their...
Ch. 4.4 - Prob. 4.11ECh. 4.4 - Analysis of the x, y, and z coordinates of each...Ch. 4.4 - Determine the number of IR-active CO stretching...Ch. 4.4 - Prob. 4.14ECh. 4 - Determine the point groups for a. Ethane...Ch. 4 - Determine the point groups for a. Ethylene b....Ch. 4 - Determine the point groups for a. Acetylene b....Ch. 4 - Determine the point groups for a. Naphthalene b....Ch. 4 - Determine the point groups for a. 1,1’ ...Ch. 4 - Determine the point groups for a. Cyclohexane...Ch. 4 - Determine the point groups for a. A sheet of...Ch. 4 - Determine the point groups for a. A flat oval...Ch. 4 - Determine the point groups for a. A triangular...Ch. 4 - Determine the point groups for the examples of...Ch. 4 - Determine the point groups of the molecules in the...Ch. 4 - Determine the point groups of the molecules and...Ch. 4 - Determine the point groups of the following atomic...Ch. 4 - a. Show that a cube has the same symmetry elements...Ch. 4 - Suppose an octahedron can have either yellow or...Ch. 4 - What point groups are represented by the symbols...Ch. 4 - Prob. 4.17PCh. 4 - Determine the point groups for the following flags...Ch. 4 - Prepare a representation flowchart according to...Ch. 4 - For trans-1,2-dichloroethylene, which has C2h...Ch. 4 - Ethylene has D2h symmetry. a. List all the...Ch. 4 - Using the D2d character table, a. Determine the...Ch. 4 - Reduce the following representations to...Ch. 4 - For D4h symmetry use sketches to show that dxy...Ch. 4 - Prob. 4.25PCh. 4 - XeOF4 has one of the more interesting structures...Ch. 4 - Repeat the procedure from the previous problem,...Ch. 4 - For the following molecules, determine the number...Ch. 4 - Prob. 4.29PCh. 4 - The structure of 1,1,2,2-tetraiododisilane is...Ch. 4 - Both cis and trans isomers of IO2F4 have been...Ch. 4 - White elemental phosphorus consists of tetrahedral...Ch. 4 - Complexes of the general formula Fe(CO)5x( PR3)x...Ch. 4 - Prob. 4.35PCh. 4 - Prob. 4.36PCh. 4 - Prob. 4.37PCh. 4 - Prob. 4.38PCh. 4 - Determine the point groups of the following...Ch. 4 - Prob. 4.40PCh. 4 - Determine the point groups of the following: a....Ch. 4 - Use the Internet to search for molecules with the...
Additional Science Textbook Solutions
Find more solutions based on key concepts
1.1 Write a one-sentence definition for each of the following:
a. chemistry
b. chemical
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
Draw structures for a saturated hydrocarbon that has a molecular ion with an m/z value of 128.
Organic Chemistry (8th Edition)
16.110 Calculate the molar solubility of in:
(a) Pure water (b)
Chemistry (7th Edition)
The following reaction has a value of G = 2.1kJ/mol(0.50kcaI/mol). CH3Br + H2S CH3 SH + HBr a. Calculate Keq a...
Organic Chemistry (9th Edition)
If you have an aqueous solution that contains 1.5 moles of HCI, how many moles of ions are in the solution?
a...
Chemistry: The Central Science (14th Edition)
Q1. What is the empirical formula of a compound with the molecular formula
Chemistry: A Molecular Approach (4th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Using infrared spectroscopy, how could you experimentally determine that you have the trans isomer rather than the cis one?arrow_forwardNMR theory: Spin-lattice (T,) relaxation refers to the amount of time required for the spins to return to their thermal equilibrium distribution along the xy plane. * O True Falsearrow_forwardINORGANIC Magnetic susceptibility: Compare the two compounds spin state- how can these two compounds be compare and contrast? Product µeff µs [(Me5dien)CoCl2 4.43 BM 3.87 BM [Me5dien)Co(SCN)2] 4.26 BM 3.87 BMarrow_forward
- Maximum covalence of Nitrogen is ‘4’ but the heavier elements of group15 show covalence greater than ‘4’. Why?arrow_forwardThe IR frequency of C≡N¯ in fac-[IrCl3(C≡N)3] is 2200 cm-1. Estimate the IR frequency of C≡N¯ for fac-[IrF3(C≡N)3] with an explanation.arrow_forwardThe infrared spectrum of CBr4 has a strong absorption at 667 cm-1. What is the correct assignment of this absorption?and why ? a.) A bending or bending mode. b.) The asymmetric stretching of the four C-Br bonds. c.) The symmetric stretching of the four C-Br bonds. d.) The stretching of a C-Br bond.arrow_forward
- What type(s) of molecular motion is (are) observed using infrared spectroscopy? A. Stretching and bending B. Rotation and excitation C. Spin flipping. D. Fragmentationarrow_forward2. Do you observe any change in the form of the absorbance bands while moving from nonpolar to polar solvents?arrow_forwardWhat are 2 most unique aspect of NMR, UV spectroscopy , IR spectroscopy, and Mass Spectroscopyarrow_forward
- Build MOs of Co(CO)4C12 for only sigma bonds with D4h isomer. Then, find one mode of vibration that is IR active. CS Scanned with CamScannerarrow_forwardWhich of the following molecules would be expected to have absorbance in the IR range from an asymmetric stretching vibration? CO2 CF4 CCl4 NO2 All of the above.arrow_forwardIf ß=-260 kJ/mol, what wavelength of light can excite the lowest electronic transition of the linear butadiene molecule? O 371 nm O 875 nm O 532 nm O 1064 nm O 113 nmarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Photochemistry : Introduction to Basic Theory of Photochemical Process [Part 1]; Author: Dr. Vikrant Palekar;https://www.youtube.com/watch?v=2NDOL11d6no;License: Standard YouTube License, CC-BY
Photochemistry-1; Author: CH-08:ARYABHATT [Mathematics, Physics, Chemistry];https://www.youtube.com/watch?v=DC4J0t1z3e8;License: Standard Youtube License