A student set up a Cu-Fe cell according to the procedure in Part 2. They measured the cell voltage twice at 298 K and determined the average of their measurements to be 0.682 V. Determine what the concentration of the Fe2+(aq) solution was as it should have changed due to auto-oxidation. Report your answer, in mol/L, to the appropriate number of significant figures and only report the numerical value (no units). Use the special "e" notation to answer this question. If your answer is one significant figure, do not include a decimal place (ie. 7e2 not 7.e2).
A student set up a Cu-Fe cell according to the procedure in Part 2. They measured the cell voltage twice at 298 K and determined the average of their measurements to be 0.682 V. Determine what the concentration of the Fe2+(aq) solution was as it should have changed due to auto-oxidation. Report your answer, in mol/L, to the appropriate number of significant figures and only report the numerical value (no units). Use the special "e" notation to answer this question. If your answer is one significant figure, do not include a decimal place (ie. 7e2 not 7.e2).
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter17: Electrochemistry And Its Applications
Section: Chapter Questions
Problem 92QRT
Related questions
Question
Please help me, i need fast solution .
Please don't provide handwritten solution .....
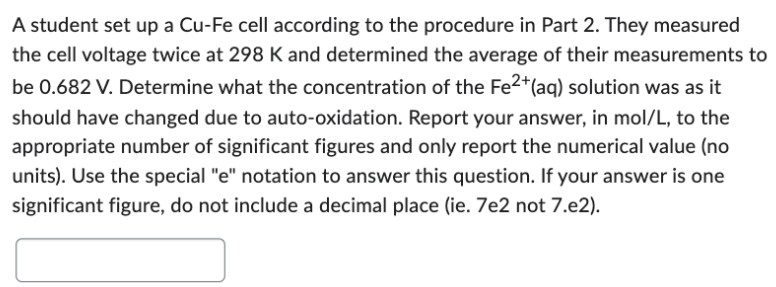
Transcribed Image Text:A student set up a Cu-Fe cell according to the procedure in Part 2. They measured
the cell voltage twice at 298 K and determined the average of their measurements to
be 0.682 V. Determine what the concentration of the Fe2+ (aq) solution was as it
should have changed due to auto-oxidation. Report your answer, in mol/L, to the
appropriate number of significant figures and only report the numerical value (no
units). Use the special "e" notation to answer this question. If your answer is one
significant figure, do not include a decimal place (ie. 7e2 not 7.e2).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning



Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning