rand name of soft drink oncentration of undiluted standard phosphate solution, M 3x/03 M Solution Number %T Absorbance 1 15%0 1.6 2 3% 1.5 Phosphate Concentration, mol/L 6.00 x 10-5 9.00x 10-5 3 1% 1. S 4 0.5% 1.20 x 10-5 1.50x10-5 31% 0.510 * 2.3 x 10-5 oft drink (dilute) concentration from Beer's law plot. See Figure 2. culations a. Show your setup for the calculation of the concentration of the phosphate ion in Solution 1. M₂ = M₁V₁ Vz 03.00x 103 M) C0.002002) =6.00×105 0.1000 L b. Show your calculation for the concentration of phosphoric acid in the original (undiluted) soft drink. Hint: Use the formula M,V₁ = M2V2 to solve for M₁. Concentration of phosphoric acid in original (undiluted) soft drink, M
rand name of soft drink oncentration of undiluted standard phosphate solution, M 3x/03 M Solution Number %T Absorbance 1 15%0 1.6 2 3% 1.5 Phosphate Concentration, mol/L 6.00 x 10-5 9.00x 10-5 3 1% 1. S 4 0.5% 1.20 x 10-5 1.50x10-5 31% 0.510 * 2.3 x 10-5 oft drink (dilute) concentration from Beer's law plot. See Figure 2. culations a. Show your setup for the calculation of the concentration of the phosphate ion in Solution 1. M₂ = M₁V₁ Vz 03.00x 103 M) C0.002002) =6.00×105 0.1000 L b. Show your calculation for the concentration of phosphoric acid in the original (undiluted) soft drink. Hint: Use the formula M,V₁ = M2V2 to solve for M₁. Concentration of phosphoric acid in original (undiluted) soft drink, M
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter15: Solutions
Section: Chapter Questions
Problem 62QAP
Related questions
Question
Just needing help on part b please.

Transcribed Image Text:12. Use the dilution equation:
M₁V₁ = M₂V₂
to calculate the concentration of the phosphate ion in the solutions you used for the Beer's
law plot. For example, if a 2.00 mL sample (V₁) of 3.00 x 10-3 M (M₁) standard phosphate
solution is diluted to 100.00 mL (V2) in a volumetric flask, the concentration of the re-
sulting solution would be given by the following:
M₁V, (3.00×10-³M)(0.00200 L)
M₁ =
V₂
0.1000 L
= 6.00 × 10-5M *
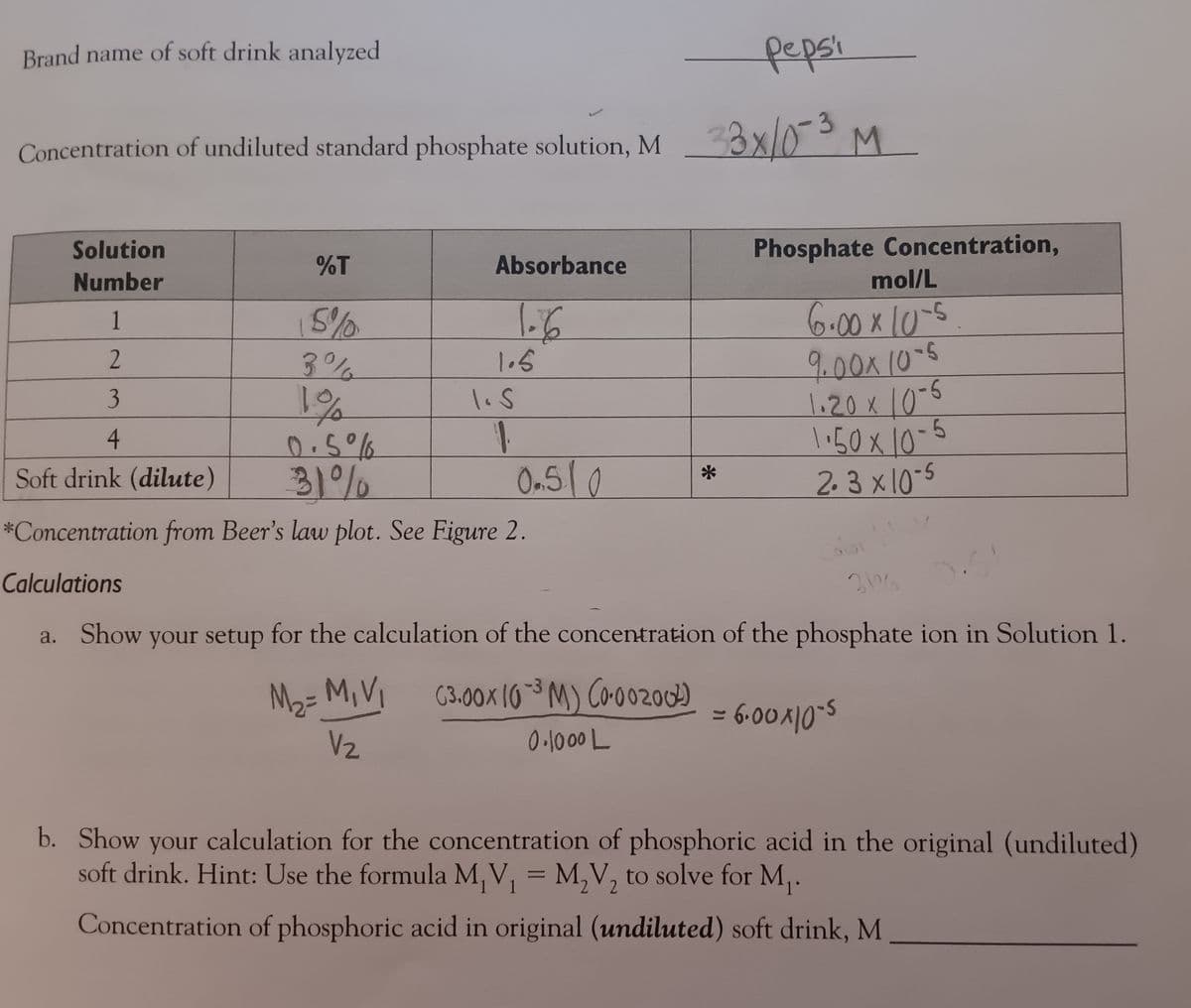
Transcribed Image Text:Brand name of soft drink analyzed
Concentration of undiluted standard phosphate solution, M
Pepsi
M 33x/0-3
M
Solution
Number
%T
Absorbance
1
15%
1.6
2
3%
1.5
3
1%
1. S
0.5%
1
31%
0.510
4
Soft drink (dilute)
*Concentration from Beer's law plot. See Figure 2.
Calculations
*
Phosphate Concentration,
mol/L
6.00 x 10-5
9.00 x 10-5
1.20 x 10-5
1·50 × 10-5
1.50×10
2.3 x 10-5
Colo
3107
a. Show your setup for the calculation of the concentration of the phosphate ion in Solution 1.
M₂ = M, V₁
V₂
(3.00 x 10-3 M) (0.002002)
0.1000 L
1
= 6.00×105
b. Show your calculation for the concentration of phosphoric acid in the original (undiluted)
soft drink. Hint: Use the formula M₁V₁ = M₂V₂ to solve for M₁.
2
Concentration of phosphoric acid in original (undiluted) soft drink, M
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 7 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning