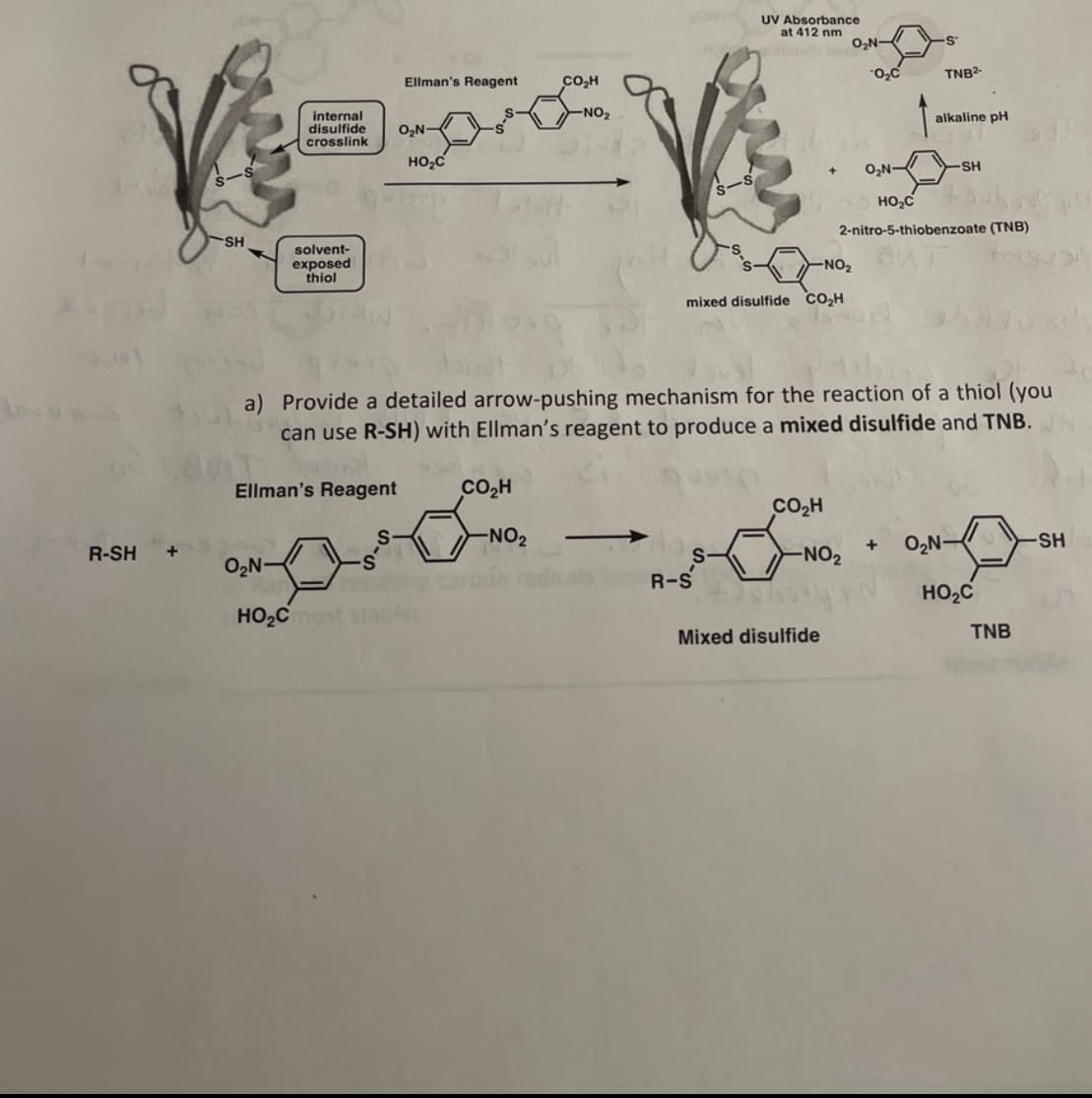
SH internal Ellman's Reagent CO₂H disulfide crosslink O₂N- HO₂C solvent- exposed thiol UV Absorbance at 412 nm O₂N- O₂C TNB2- alkaline pH + O₂N- -SH 2-nitro-5-thiobenzoate (TNB) -NO₂ mixed disulfide CO₂H a) Provide a detailed arrow-pushing mechanism for the reaction of a thiol (you can use R-SH) with Ellman's reagent to produce a mixed disulfide and TNB. Ellman's Reagent CO₂H S -NO2 - R-SH + O₂N- S HO₂Cmo CO₂H S + NO2 O₂N- SH R-S HO₂C Mixed disulfide TNB
SH internal Ellman's Reagent CO₂H disulfide crosslink O₂N- HO₂C solvent- exposed thiol UV Absorbance at 412 nm O₂N- O₂C TNB2- alkaline pH + O₂N- -SH 2-nitro-5-thiobenzoate (TNB) -NO₂ mixed disulfide CO₂H a) Provide a detailed arrow-pushing mechanism for the reaction of a thiol (you can use R-SH) with Ellman's reagent to produce a mixed disulfide and TNB. Ellman's Reagent CO₂H S -NO2 - R-SH + O₂N- S HO₂Cmo CO₂H S + NO2 O₂N- SH R-S HO₂C Mixed disulfide TNB
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter9: Atomic Absorption And Atomic Fluorescence Spectrometry
Section: Chapter Questions
Problem 9.6QAP
Related questions
Question
Transcribed Image Text:R-SH
+
SH
Ellman's Reagent
CO₂H
-NO2
internal
disulfide
crosslink
O₂N-
HO₂C
solvent-
exposed
thiol
UV Absorbance
at 412 nm
O₂N-
TNB2-
alkaline pH
+
O₂N-
-SH
2-nitro-5-thiobenzoate (TNB)
-NO₂
mixed disulfide CO₂H
a) Provide a detailed arrow-pushing mechanism for the reaction of a thiol (you
can use R-SH) with Ellman's reagent to produce a mixed disulfide and TNB.
Ellman's Reagent
O₂N-
CO₂H
-NO2
S
CO₂H
།་,་༼་་
HO2Cmos
R-S
+
-NO2
O₂N-
SH
HO₂C
TNB
Mixed disulfide
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
