2.6 a) For the reaction: AH=-918 kJ and AS --112 JK-¹ Why is the entropy change negative? №2(g) + 3H2(g)=2 NH3(g) b) Show that the reaction is spontaneous at 25 °C. c) Even though the reaction is spontaneous, a catalyst is required before any ammonia will be formed. Explain why a catalyst is required.
2.6 a) For the reaction: AH=-918 kJ and AS --112 JK-¹ Why is the entropy change negative? №2(g) + 3H2(g)=2 NH3(g) b) Show that the reaction is spontaneous at 25 °C. c) Even though the reaction is spontaneous, a catalyst is required before any ammonia will be formed. Explain why a catalyst is required.
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter17: Chemcial Thermodynamics
Section: Chapter Questions
Problem 17.78QE
Related questions
Question
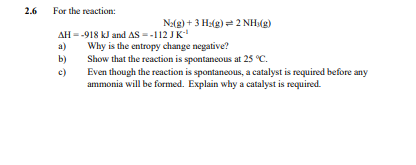
Transcribed Image Text:2.6
a)
For the reaction:
AH=-918 kJ and AS --112 JK-¹
Why is the entropy change negative?
№2(g) + 3H2(g)=2 NH3(g)
b)
Show that the reaction is spontaneous at 25 °C.
c)
Even though the reaction is spontaneous, a catalyst is required before any
ammonia will be formed. Explain why a catalyst is required.
AI-Generated Solution
Unlock instant AI solutions
Tap the button
to generate a solution
Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning