If 29.0 mL of 1.7 M C2H5NH2 are titrated with 0.8 M HCI, what is the pH at 13.8 mL? Answer: Exactly 34.9 mL of 0.2 M HNO2 are titrated with a 0.2 M NaOH solution. What is the pH at the equivalence point? Answer:
If 29.0 mL of 1.7 M C2H5NH2 are titrated with 0.8 M HCI, what is the pH at 13.8 mL? Answer: Exactly 34.9 mL of 0.2 M HNO2 are titrated with a 0.2 M NaOH solution. What is the pH at the equivalence point? Answer:
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter15: Additional Aqueous Equilibria
Section: Chapter Questions
Problem 93QRT: When 40.00 mL of a weak monoprotic acid solution is titrated with 0.100-M NaOH, the equivalence...
Related questions
Question
I need both solution
Please don't provide handwritten solution
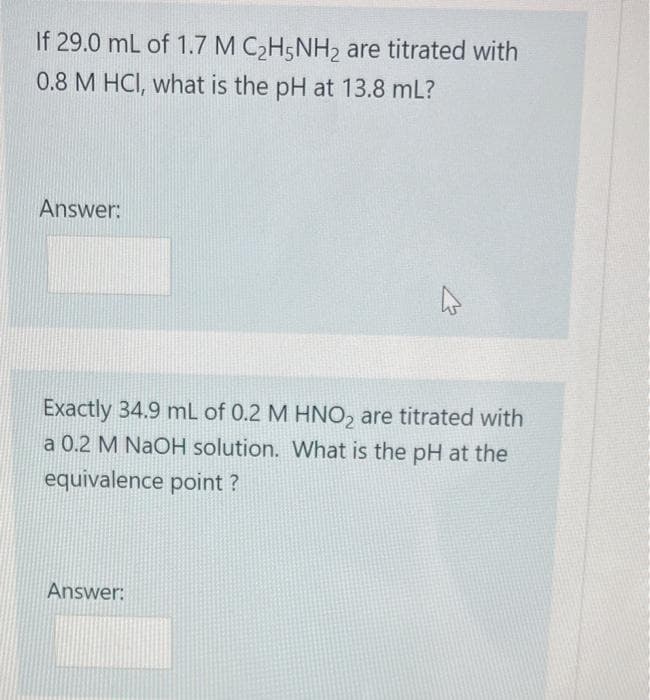
Transcribed Image Text:If 29.0 mL of 1.7 M C2H5NH2 are titrated with
0.8 M HCI, what is the pH at 13.8 mL?
Answer:
Exactly 34.9 mL of 0.2 M HNO2 are titrated with
a 0.2 M NaOH solution. What is the pH at the
equivalence point?
Answer:
AI-Generated Solution
Unlock instant AI solutions
Tap the button
to generate a solution
Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole



Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning