Often in the lab, you are given a stock solution that you must dilute before you can use in the lab. For instance, HNO comes as 18.3 M. There are serious biological/safety hazards with using 18.3 M HNO, (it can give you chemical burns within seconds of contact), but it can make a lot of solutions for laboratory use. Usually, we use 1 M HNO, in chemistry class. You will figure out how to do this dilution calculation and many others using a formula that chemists use daily: 1) You add 1.00 Liter of H2O to a 2.00 Liter, 5.00M solution of HCI. What is the concentration of this new solution? 2) How much 18.3 M HNO, is needed to make 1.0 L of 1.0 M HNO₂?
Often in the lab, you are given a stock solution that you must dilute before you can use in the lab. For instance, HNO comes as 18.3 M. There are serious biological/safety hazards with using 18.3 M HNO, (it can give you chemical burns within seconds of contact), but it can make a lot of solutions for laboratory use. Usually, we use 1 M HNO, in chemistry class. You will figure out how to do this dilution calculation and many others using a formula that chemists use daily: 1) You add 1.00 Liter of H2O to a 2.00 Liter, 5.00M solution of HCI. What is the concentration of this new solution? 2) How much 18.3 M HNO, is needed to make 1.0 L of 1.0 M HNO₂?
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter11: Solutions
Section: Chapter Questions
Problem 65AP
Related questions
Question
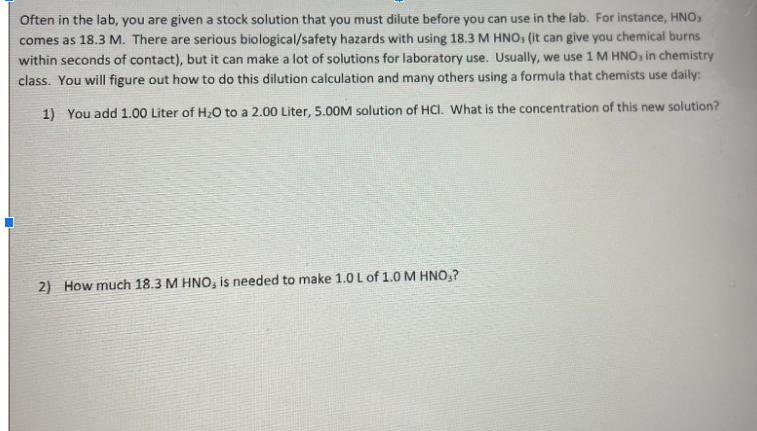
Transcribed Image Text:Often in the lab, you are given a stock solution that you must dilute before you can use in the lab. For instance, HNO
comes as 18.3 M. There are serious biological/safety hazards with using 18.3 M HNO, (it can give you chemical burns
within seconds of contact), but it can make a lot of solutions for laboratory use. Usually, we use 1 M HNO, in chemistry
class. You will figure out how to do this dilution calculation and many others using a formula that chemists use daily:
1) You add 1.00 Liter of H2O to a 2.00 Liter, 5.00M solution of HCI. What is the concentration of this new solution?
2) How much 18.3 M HNO, is needed to make 1.0 L of 1.0 M HNO₂?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 1 steps

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning