0.0517 mols of an ideal gas is quickly compressed from 1.12 L to 0.410 L . Initially the gas has a pressure of 1.49 atm. The molecules makin the gas have 5 degrees of freedom. Part 1) How much heat is added during this compression? Q = Part 2) What is the change in the internal energy of the gas during this compression? ΔΕ J Part 3) How much work is done on the gas during this compression? W = J
0.0517 mols of an ideal gas is quickly compressed from 1.12 L to 0.410 L . Initially the gas has a pressure of 1.49 atm. The molecules makin the gas have 5 degrees of freedom. Part 1) How much heat is added during this compression? Q = Part 2) What is the change in the internal energy of the gas during this compression? ΔΕ J Part 3) How much work is done on the gas during this compression? W = J
Related questions
Question
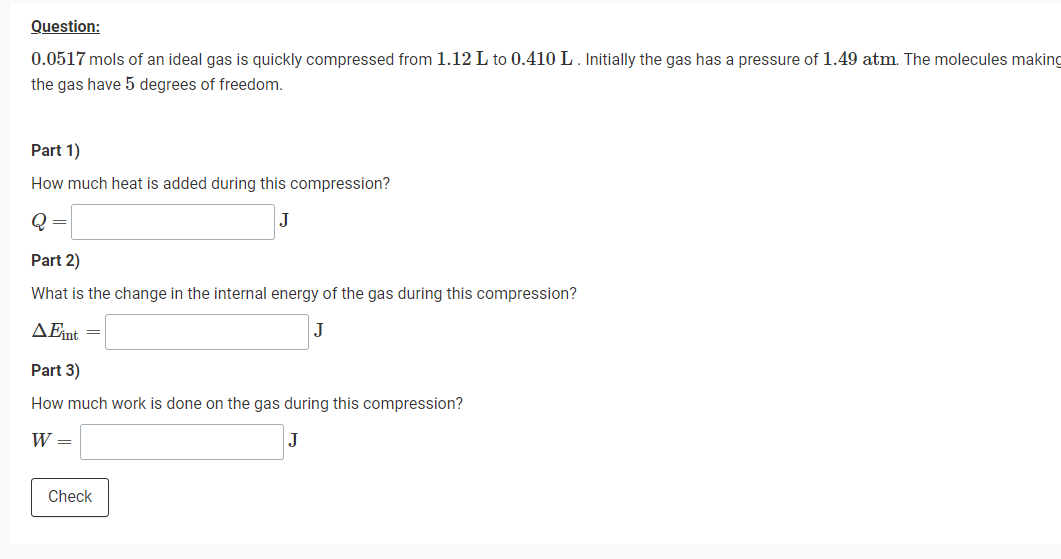
Transcribed Image Text:Question:
0.0517 mols of an ideal gas is quickly compressed from 1.12 L to 0.410 L. Initially the gas has a pressure of 1.49 atm. The molecules making
the gas have 5 degrees of freedom.
Part 1)
How much heat is added during this compression?
Q
Part 2)
What is the change in the internal energy of the gas during this compression?
AFnt
Part 3)
How much work is done on the gas during this compression?
W =
J
Check
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps
