1 A metal ball of mass 0.1 kg is heated upto 500°C and dropped into a vessel of heat capacity 800 JK¯¹ and containing 0.5 kg water. The initial temperature of water and vessel is 30°C. What is the approximate percentage increment in the temperature of the water? [Take, specific heat capacities of water and metal are respectively 4200 Jkg ¹K-¹ and 400 Jkg ¹K¹] (a) 25% (b) 15% (c) 30% (d) 20%
1 A metal ball of mass 0.1 kg is heated upto 500°C and dropped into a vessel of heat capacity 800 JK¯¹ and containing 0.5 kg water. The initial temperature of water and vessel is 30°C. What is the approximate percentage increment in the temperature of the water? [Take, specific heat capacities of water and metal are respectively 4200 Jkg ¹K-¹ and 400 Jkg ¹K¹] (a) 25% (b) 15% (c) 30% (d) 20%
Related questions
Question
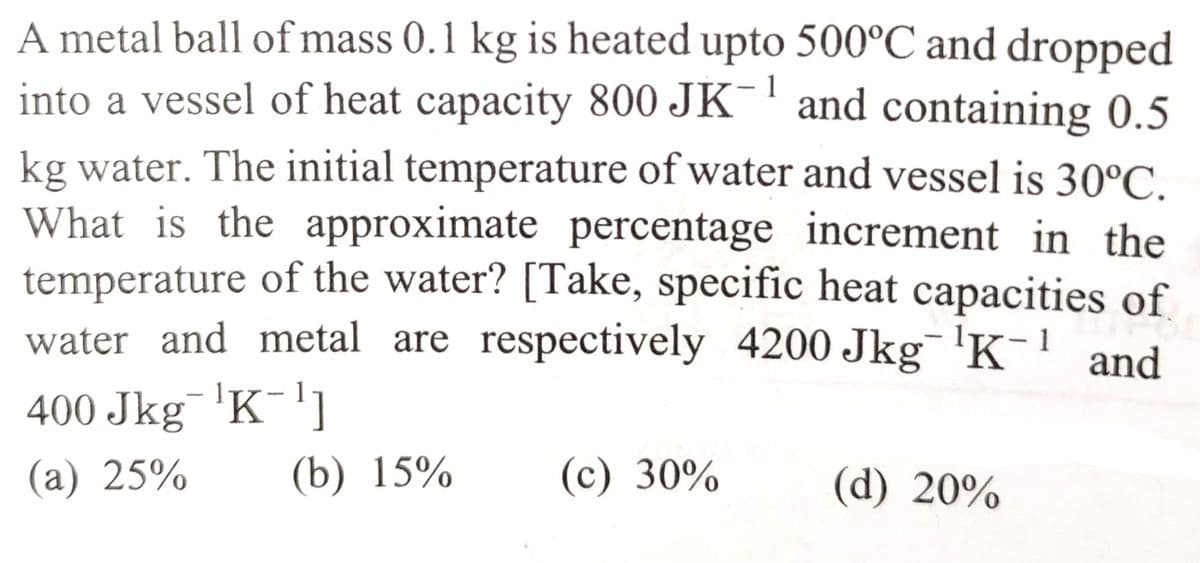
Transcribed Image Text:1
A metal ball of mass 0.1 kg is heated upto 500°C and dropped
into a vessel of heat capacity 800 JK and containing 0.5
kg water. The initial temperature of water and vessel is 30°C.
What is the approximate percentage increment in the
temperature of the water? [Take, specific heat capacities of
water and metal are respectively 4200 Jkg ¹K-¹ and
400 Jkg¯¹K-¹1
(a) 25%
(b) 15%
(c) 30%
(d) 20%
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps
