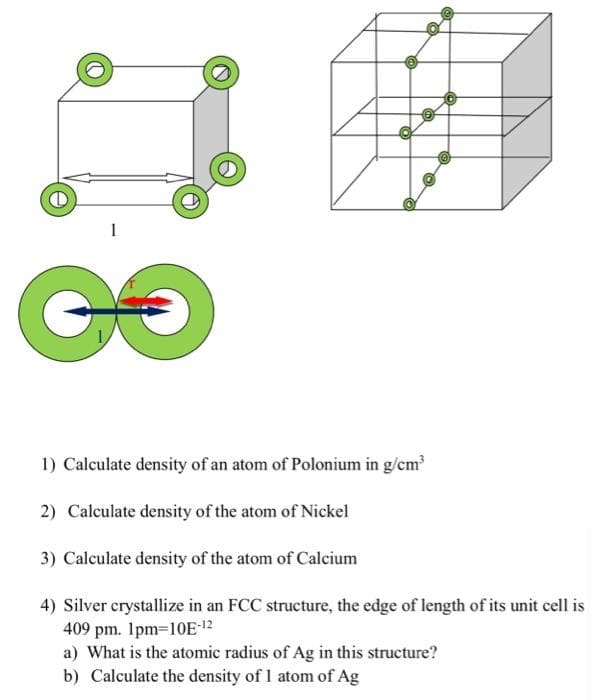
1 GO 1) Calculate density of an atom of Polonium in g/cm 2) Calculate density of the atom of Nickel 3) Calculate density of the atom of Calcium 4) Silver crystallize in an FCC structure, the edge of length of its unit cell is 409 pm. Ipm=10E-12 a) What is the atomic radius of Ag in this structure? b) Calculate the density of 1 atom of Ag
1 GO 1) Calculate density of an atom of Polonium in g/cm 2) Calculate density of the atom of Nickel 3) Calculate density of the atom of Calcium 4) Silver crystallize in an FCC structure, the edge of length of its unit cell is 409 pm. Ipm=10E-12 a) What is the atomic radius of Ag in this structure? b) Calculate the density of 1 atom of Ag
Welding: Principles and Applications (MindTap Course List)
8th Edition
ISBN:9781305494695
Author:Larry Jeffus
Publisher:Larry Jeffus
Chapter26: Welding Metallurgy
Section: Chapter Questions
Problem 7R
Related questions
Question
Transcribed Image Text:GO
1) Calculate density of an atom of Polonium in g/cm
2) Calculate density of the atom of Nickel
3) Calculate density of the atom of Calcium
4) Silver crystallize in an FCC structure, the edge of length of its unit cell is
409 pm. 1pm=10E-12
a) What is the atomic radius of Ag in this structure?
b) Calculate the density of 1 atom of Ag

Transcribed Image Text:Recall:
For a sphere: v= 4/3IIr³
Number of Avogadro : 6,02 10E²³ atom
þ polonium =202 g/mol
I polonium = 336 10E_1²m
þ Nickel =58,69 g/mol
I Nickel = 0,3524 nm
þ calcium =40,078 g/mol
1 calcium = 558,8 10E_12 m
þ silver =107,86 g/mol
1 silver = 409 10E_12 m
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps

Recommended textbooks for you

Welding: Principles and Applications (MindTap Cou…
Mechanical Engineering
ISBN:
9781305494695
Author:
Larry Jeffus
Publisher:
Cengage Learning

Welding: Principles and Applications (MindTap Cou…
Mechanical Engineering
ISBN:
9781305494695
Author:
Larry Jeffus
Publisher:
Cengage Learning