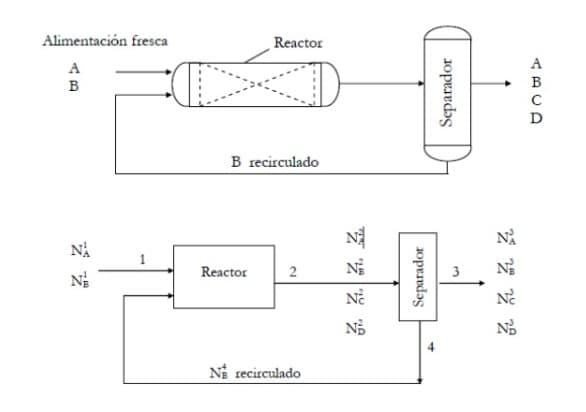
1 Reactor 2 Na recirculado ZZZ2 Separador 4 22222
The reaction 2A +5B --- > 3C + 6D
Occurs in a reactor where fresh substance A is present with a 30% excess over the stoichiometric amount needed to react with the fresh B. The overall conversion of B in the process is 90% and the one-step conversion of B is 60%. Most of B that do not reacted is recovered in a separator and recirculated to the reactor. The fresh feed to the reactor contains A and B. . Use advance method of the reaction or rate of reaction. Calculate the unknown flows to produce 300 moles/h of C. Construct a table of degrees of freedom, determine solution sequence and validate results with checking. Use reaction progress or reaction rate method
Relations: R1: Conversion of B in the reactor of 60%
A2: Overall conversion of B is 95%
R3: N1 A is fed 30% in excess of the theoretical for react with N1 b.
Step by step
Solved in 2 steps with 2 images









