1.) A Carnot engine is coupled to a Carnot refrigerator so that all the work produced by the engine is used by the refrigerator in extraction of heat from a heat reservoir at 270K at the rate of 4 kJ/s. The source of energy for the Carnot engine is a heat reservoir at 500K. If both devise discard heat to the surrounding at 300K, a. How much heat does the engine absorb from the 500K reservoir? b. If the actual efficiency of the engine is n= Ncarnot /1.5 and the COP = COPCarnot/1.5, how much heat does the engine absorb from the 5O0K reservoir?
1.) A Carnot engine is coupled to a Carnot refrigerator so that all the work produced by the engine is used by the refrigerator in extraction of heat from a heat reservoir at 270K at the rate of 4 kJ/s. The source of energy for the Carnot engine is a heat reservoir at 500K. If both devise discard heat to the surrounding at 300K, a. How much heat does the engine absorb from the 500K reservoir? b. If the actual efficiency of the engine is n= Ncarnot /1.5 and the COP = COPCarnot/1.5, how much heat does the engine absorb from the 5O0K reservoir?
Refrigeration and Air Conditioning Technology (MindTap Course List)
8th Edition
ISBN:9781305578296
Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Chapter44: Geothermal Heat Pumps
Section: Chapter Questions
Problem 13RQ: If 14 gpm of water is flowing through an open-loop heat pump with a temperature difference of 7F,...
Related questions
Question
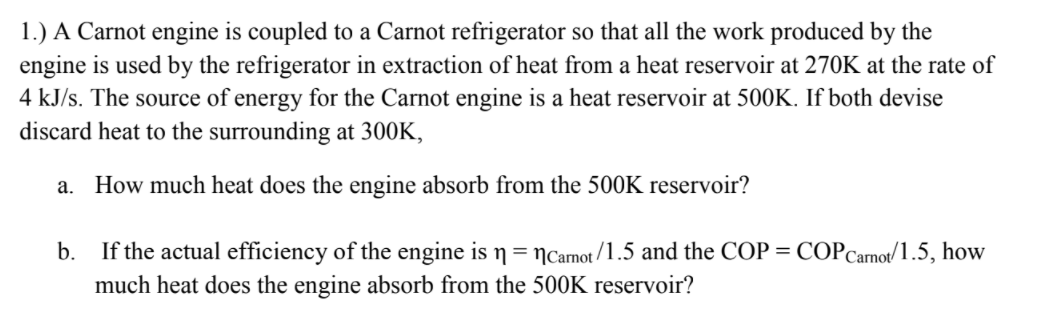
Transcribed Image Text:1.) A Carnot engine is coupled to a Carnot refrigerator so that all the work produced by the
engine is used by the refrigerator in extraction of heat from a heat reservoir at 270K at the rate of
4 kJ/s. The source of energy for the Carnot engine is a heat reservoir at 500K. If both devise
discard heat to the surrounding at 300K,
a. How much heat does the engine absorb from the 500K reservoir?
b. If the actual efficiency of the engine is n= Ncarnot /1.5 and the COP = COPCarnot/1.5, how
much heat does the engine absorb from the 5O0K reservoir?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Refrigeration and Air Conditioning Technology (Mi…
Mechanical Engineering
ISBN:
9781305578296
Author:
John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:
Cengage Learning

Refrigeration and Air Conditioning Technology (Mi…
Mechanical Engineering
ISBN:
9781305578296
Author:
John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:
Cengage Learning