1. According to molecular orbital theory we may construct a ols bonding and antibonding molecular orbital from 1s atomic orbitals on center A and center B of H . (a) Sketch y and Iy? for the bonding and antibonding orbitals B A Ival?- A (b) write the Hamiltonian for this system (easiest in atomic units). FIGURE 9.2 Definition of the distances involved in the Hamiltonian operator for H; (Equation 9.4). (c) Write expressions for the bonding and antibonding energies in terms of the energy of a 1s atomic orbital, the coulombic integral and the exchange integral.
1. According to molecular orbital theory we may construct a ols bonding and antibonding molecular orbital from 1s atomic orbitals on center A and center B of H . (a) Sketch y and Iy? for the bonding and antibonding orbitals B A Ival?- A (b) write the Hamiltonian for this system (easiest in atomic units). FIGURE 9.2 Definition of the distances involved in the Hamiltonian operator for H; (Equation 9.4). (c) Write expressions for the bonding and antibonding energies in terms of the energy of a 1s atomic orbital, the coulombic integral and the exchange integral.
Related questions
Question
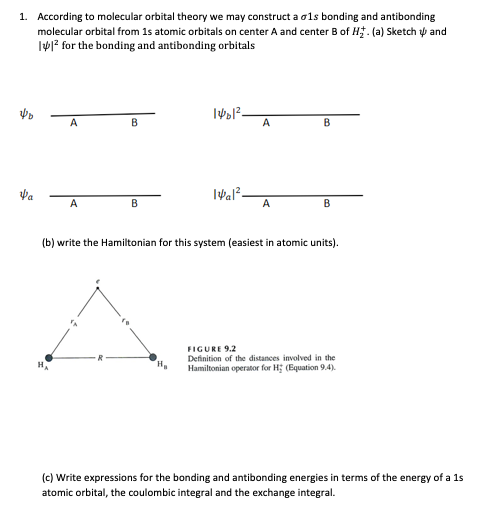
Transcribed Image Text:1. According to molecular orbital theory we may construct a ols bonding and antibonding
molecular orbital from 1s atomic orbitals on center A and center B of H. (a) Sketch ý and
IV? for the bonding and antibonding orbitals
A
A
B
Va
A
A
В
(b) write the Hamiltonian for this system (easiest in atomic units).
FIGURE 9.2
Definition of the distances involved in the
Hamiltonian operator for H; (Equation 9.4).
(c) Write expressions for the bonding and antibonding energies in terms of the energy of a 1s
atomic orbital, the coulombic integral and the exchange integral.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images
