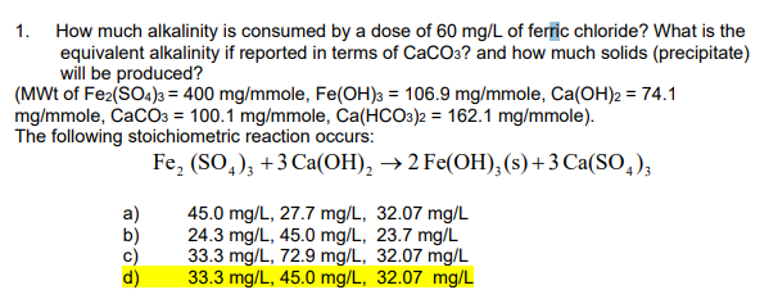
1. How much alkalinity is consumed by a dose of 60 mg/L of ferric chloride? What is the equivalent alkalinity if reported in terms of CaCO3? and how much solids (precipitate) will be produced? (MWt of Fe2(SO4)3 = 400 mg/mmole, Fe(OH)3 = 106.9 mg/mmole, Ca(OH)2 = 74.1 mg/mmole, CaCO3 = 100.1 mg/mmole, Ca(HCO3)2 = 162.1 mg/mmole). The following stoichiometric reaction occurs: Fe₂(SO4)3 +3 Ca(OH)₂ → 2 Fe(OH)3 (s) + 3 Ca(SO4)3 a) 45.0 mg/L, 27.7 mg/L, 32.07 mg/L 24.3 mg/L, 45.0 mg/L, 23.7 mg/L 33.3 mg/L, 72.9 mg/L, 32.07 mg/L 33.3 mg/L, 45.0 mg/L, 32.07 mg/L
Basic Terminologies
Terminology is a systematic study for the implementation of the terms or words and their correspondence within their respective domain of human activity in the case of multilingual (more than 2) or bilingual (2) languages. Terminology is defined as the study of concepts, conceptual related phrases or expressions, and lexicography, on the other hand, is the discipline used to study any type of word and its meaning. Technical ventures and institutes create and gather their resources or word collection also known as a Glossary.
Common Units Of Pressure
Pressure is something that we experience in daily life but fail to recognize it as our body is so accustomed to it. For example, the atmospheric air applies pressure on our body, but we don’t feel it as we are exposed to it since birth and our body is habitual to it. When we write using a pen, we need to apply some pressure over the pen to make it start writing. But these are our day-to-day activity which we fail to recognize
Answer is D, wanting to know how to calculate.
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images









