1. Problem Description: The total nuclear binding energy is the energy required to split a nucleus of an atom in its component parts: protons and neutrons, or, collectively, the nucleons. It describes how strongly nucleons are bound to each other. When a high amount of energy is needed to separate the nucleons, it means nucleus is very stable and the neutrons and protons are tightly bound to each other. The atomic number or proton number (symbol Z) is the number of protons found in the nucleus of an atom. The sum of the atomic number Z and the number of neutrons N gives the mass number A of an atom. + Binding energy - Separated nucleons (greater mass) Nucleus (smaller mass) Figure 1: Binding Energy in the Nucleus The approximate nuclear binding energy Eb in million electron volts, of an atomic nucleus with atomic number Z and mass number A is calculated using the following formula: - azA3 – az1- a4° (A – 2Z)² ¸ a5 Eb = a,A – A A3 where, a, = 15.67,az = 17.23, az = 0.75, a4 = 93.2 ,and %3D
1. Problem Description: The total nuclear binding energy is the energy required to split a nucleus of an atom in its component parts: protons and neutrons, or, collectively, the nucleons. It describes how strongly nucleons are bound to each other. When a high amount of energy is needed to separate the nucleons, it means nucleus is very stable and the neutrons and protons are tightly bound to each other. The atomic number or proton number (symbol Z) is the number of protons found in the nucleus of an atom. The sum of the atomic number Z and the number of neutrons N gives the mass number A of an atom. + Binding energy - Separated nucleons (greater mass) Nucleus (smaller mass) Figure 1: Binding Energy in the Nucleus The approximate nuclear binding energy Eb in million electron volts, of an atomic nucleus with atomic number Z and mass number A is calculated using the following formula: - azA3 – az1- a4° (A – 2Z)² ¸ a5 Eb = a,A – A A3 where, a, = 15.67,az = 17.23, az = 0.75, a4 = 93.2 ,and %3D
Computer Networking: A Top-Down Approach (7th Edition)
7th Edition
ISBN:9780133594140
Author:James Kurose, Keith Ross
Publisher:James Kurose, Keith Ross
Chapter1: Computer Networks And The Internet
Section: Chapter Questions
Problem R1RQ: What is the difference between a host and an end system? List several different types of end...
Related questions
Question
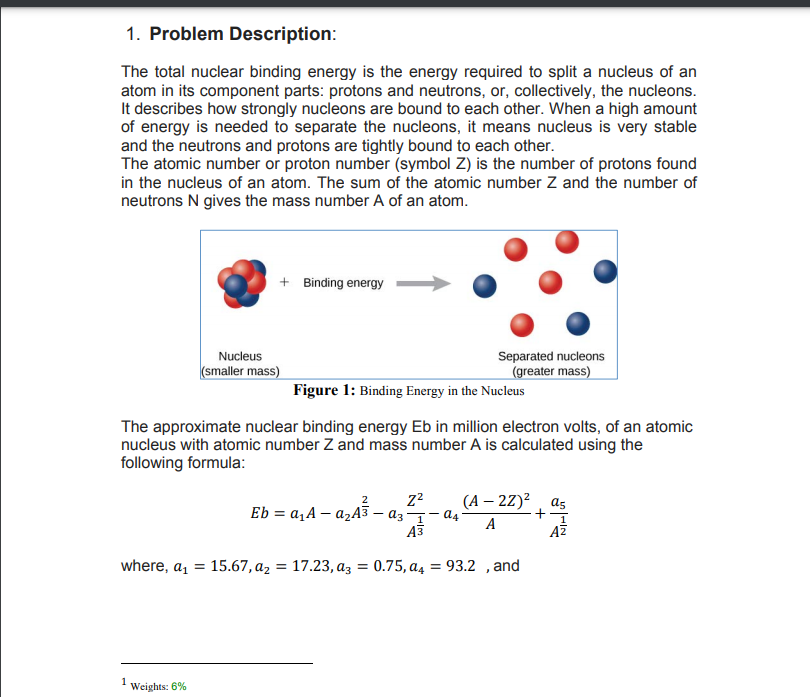
Transcribed Image Text:1. Problem Description:
The total nuclear binding energy is the energy required to split a nucleus of an
atom in its component parts: protons and neutrons, or, collectively, the nucleons.
It describes how strongly nucleons are bound to each other. When a high amount
of energy is needed to separate the nucleons, it means nucleus is very stable
and the neutrons and protons are tightly bound to each other.
The atomic number or proton number (symbol Z) is the number of protons found
in the nucleus of an atom. The sum of the atomic number Z and the number of
neutrons N gives the mass number A of an atom.
+ Binding energy
Separated nucleons
(greater mass)
Nucleus
(smaller mass)
Figure 1: Binding Energy in the Nucleus
The approximate nuclear binding energy Eb in million electron volts, of an atomic
nucleus with atomic number Z and mass number A is calculated using the
following formula:
22
(А — 22)? , as
Eb = a,A – azA3 – az- a4-
A
A3
AZ
where, a,
15.67, az = 17.23, az = 0.75, a4 = 93.2 , and
%3D
%3D
1
Weights: 6%
![if A is odd
if A and Z are both even
if A is even and Z is Odd
a5 =
12.0
(-12.0
The binding energy per nucleon (BEN) is calculated by dividing the binding
energy (Eb) by the mass number (A).
You are asked to write a program that requests the user for a valid atomic
number (Z) then goes through all values of A from A = Z to A = 4Z. For example,
if the user inputs 5 for Z then A will be all numbers from 5 (Z) to 20 (4 * Z)
inclusive, see the example output in figure 2.
If the user enters invalid atomic number that is not between 1 and 118, the
program should give the user another chance to enter a valid input as shown in
figure 2.
Your main task is to find the nucleus with the highest binding energy per nucleon,
which corresponds to the most stable configuration (figure 2), and writes a copy
of the table to a text file named output.txt (figure3).
In [25]: runfile('/Users/hamzazidoum/Documents/2101/2101_S2021/
Programming Assignments/PA4/pa4_nuclear.py', wdir='/Users/hamzazidoum/
Documents/2101/2101_S2021/Programming Assignments/PA4')
>>Enter valid atomic number (Z) (1,118]: 0
>>Enter valid atomic number (Z) (1,118]: -120
>>Enter valid atomic number (Z) (1,118]: 200
>>Enter valid atomic number (Z) (1,118]: 5
A
binding
energy
binding_ energy
per Nuc leon
5
-448.996
-226.623
-89. 799
-37.771
8.
9.
-82.990
-3.778
47.111
-11.856
-0.472
5.235
6.423
6.386
4.584
10
11
64.228
70.245
55.009
13
14
35.952
1.794
-32.682
-78.825
2.766
0.128
-2.179
-4.927
15
16
17
18
19
-123.453
-177.641
-229.307
-289.143
-7.262
-9.869
-12.069
20
-14.457
The most stable nucleus has a mass number 10
Figure 2: Sample run of the program
123 456 7 89e
5670o](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F5101b5a6-2e37-422b-85f3-a3d6cf4a77ff%2Fc5f031ff-b647-459b-b6db-a9fea7c17560%2F3ftgdtp_processed.png&w=3840&q=75)
Transcribed Image Text:if A is odd
if A and Z are both even
if A is even and Z is Odd
a5 =
12.0
(-12.0
The binding energy per nucleon (BEN) is calculated by dividing the binding
energy (Eb) by the mass number (A).
You are asked to write a program that requests the user for a valid atomic
number (Z) then goes through all values of A from A = Z to A = 4Z. For example,
if the user inputs 5 for Z then A will be all numbers from 5 (Z) to 20 (4 * Z)
inclusive, see the example output in figure 2.
If the user enters invalid atomic number that is not between 1 and 118, the
program should give the user another chance to enter a valid input as shown in
figure 2.
Your main task is to find the nucleus with the highest binding energy per nucleon,
which corresponds to the most stable configuration (figure 2), and writes a copy
of the table to a text file named output.txt (figure3).
In [25]: runfile('/Users/hamzazidoum/Documents/2101/2101_S2021/
Programming Assignments/PA4/pa4_nuclear.py', wdir='/Users/hamzazidoum/
Documents/2101/2101_S2021/Programming Assignments/PA4')
>>Enter valid atomic number (Z) (1,118]: 0
>>Enter valid atomic number (Z) (1,118]: -120
>>Enter valid atomic number (Z) (1,118]: 200
>>Enter valid atomic number (Z) (1,118]: 5
A
binding
energy
binding_ energy
per Nuc leon
5
-448.996
-226.623
-89. 799
-37.771
8.
9.
-82.990
-3.778
47.111
-11.856
-0.472
5.235
6.423
6.386
4.584
10
11
64.228
70.245
55.009
13
14
35.952
1.794
-32.682
-78.825
2.766
0.128
-2.179
-4.927
15
16
17
18
19
-123.453
-177.641
-229.307
-289.143
-7.262
-9.869
-12.069
20
-14.457
The most stable nucleus has a mass number 10
Figure 2: Sample run of the program
123 456 7 89e
5670o
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Computer Networking: A Top-Down Approach (7th Edi…
Computer Engineering
ISBN:
9780133594140
Author:
James Kurose, Keith Ross
Publisher:
PEARSON

Computer Organization and Design MIPS Edition, Fi…
Computer Engineering
ISBN:
9780124077263
Author:
David A. Patterson, John L. Hennessy
Publisher:
Elsevier Science

Network+ Guide to Networks (MindTap Course List)
Computer Engineering
ISBN:
9781337569330
Author:
Jill West, Tamara Dean, Jean Andrews
Publisher:
Cengage Learning

Computer Networking: A Top-Down Approach (7th Edi…
Computer Engineering
ISBN:
9780133594140
Author:
James Kurose, Keith Ross
Publisher:
PEARSON

Computer Organization and Design MIPS Edition, Fi…
Computer Engineering
ISBN:
9780124077263
Author:
David A. Patterson, John L. Hennessy
Publisher:
Elsevier Science

Network+ Guide to Networks (MindTap Course List)
Computer Engineering
ISBN:
9781337569330
Author:
Jill West, Tamara Dean, Jean Andrews
Publisher:
Cengage Learning

Concepts of Database Management
Computer Engineering
ISBN:
9781337093422
Author:
Joy L. Starks, Philip J. Pratt, Mary Z. Last
Publisher:
Cengage Learning

Prelude to Programming
Computer Engineering
ISBN:
9780133750423
Author:
VENIT, Stewart
Publisher:
Pearson Education

Sc Business Data Communications and Networking, T…
Computer Engineering
ISBN:
9781119368830
Author:
FITZGERALD
Publisher:
WILEY