1. The Zeeman effect refers to the splitting of certain orbitals when the atom is placed in an external magnetic field. In the absence of an external field (and neglecting spin-orbit coupling) all the I states corresponding to a particular n state have the same energy – this phenomenon is referred to as degeneracy. A magnetic field partially "lifts" this degeneracy by causing each l 0 state to split into 21 +1 substates denoted by mi, with the modified energy given by the formula -13.6 En.m = eV + m¡UgB n2 where ug = 5.78 * 10-5eV/T. a. Sketch the n=2 and n=1 energy levels, including the splitting caused by the magnetic field and compute the energy of the photon that would be emitted when the electron makes a 2p→1s transition if the atom is in a field of 50000 T
1. The Zeeman effect refers to the splitting of certain orbitals when the atom is placed in an external magnetic field. In the absence of an external field (and neglecting spin-orbit coupling) all the I states corresponding to a particular n state have the same energy – this phenomenon is referred to as degeneracy. A magnetic field partially "lifts" this degeneracy by causing each l 0 state to split into 21 +1 substates denoted by mi, with the modified energy given by the formula -13.6 En.m = eV + m¡UgB n2 where ug = 5.78 * 10-5eV/T. a. Sketch the n=2 and n=1 energy levels, including the splitting caused by the magnetic field and compute the energy of the photon that would be emitted when the electron makes a 2p→1s transition if the atom is in a field of 50000 T
Related questions
Question
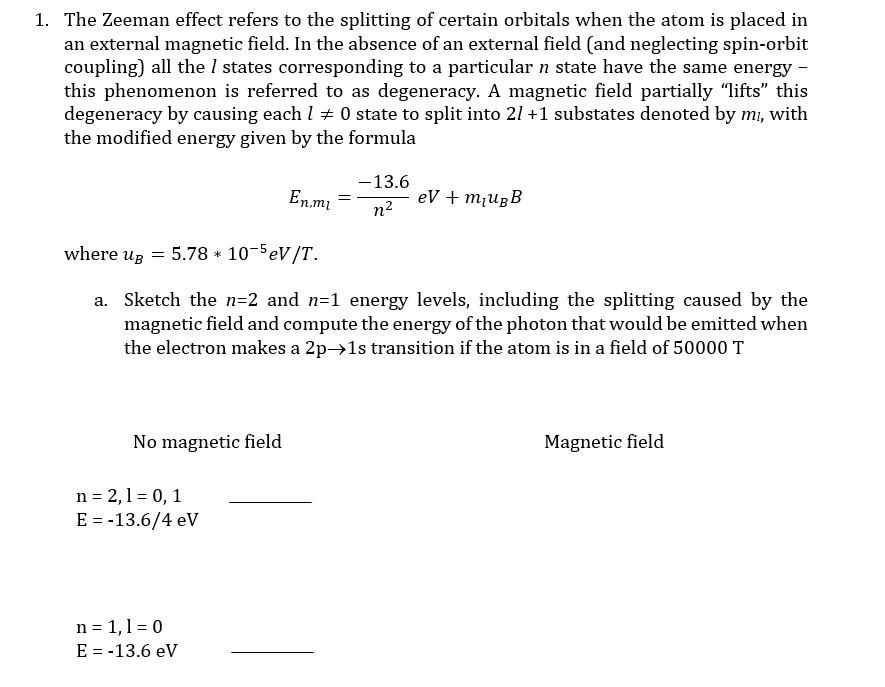
Transcribed Image Text:1. The Zeeman effect refers to the splitting of certain orbitals when the atom is placed in
an external magnetic field. In the absence of an external field (and neglecting spin-orbit
coupling) all the I states corresponding to a particular n state have the same energy –
this phenomenon is referred to as degeneracy. A magnetic field partially "lifts" this
degeneracy by causing each l 0 state to split into 21 +1 substates denoted by mi, with
the modified energy given by the formula
-13.6
En.m =
eV + m¡UgB
n2
where ug = 5.78 * 10-5eV/T.
a. Sketch the n=2 and n=1 energy levels, including the splitting caused by the
magnetic field and compute the energy of the photon that would be emitted when
the electron makes a 2p→1s transition if the atom is in a field of 50000 T
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images
