1. Two moles of helium gas are placed in a cylindrical container with a piston. The gas is at room temperature 30 °C and under a pressure of 3.3.105 Pa. When the pressure from the outside is decreased, while keeping the temperature the same as the room temperature, the volume of the ga: doubles. Use that the gas constant R=8.31 J/(mol K). Think: What kind of process is this? Isobaric, isothermal, adiabatic, isochoric or non-quasi-static? (a) Find the work the external agent does on the gas in the process. W ext. agent (b) Find the heat exchanged by the gas and indicate whether the gas takes in or gives up heat. Assume ideal gas behavior.
1. Two moles of helium gas are placed in a cylindrical container with a piston. The gas is at room temperature 30 °C and under a pressure of 3.3.105 Pa. When the pressure from the outside is decreased, while keeping the temperature the same as the room temperature, the volume of the ga: doubles. Use that the gas constant R=8.31 J/(mol K). Think: What kind of process is this? Isobaric, isothermal, adiabatic, isochoric or non-quasi-static? (a) Find the work the external agent does on the gas in the process. W ext. agent (b) Find the heat exchanged by the gas and indicate whether the gas takes in or gives up heat. Assume ideal gas behavior.
Related questions
Question
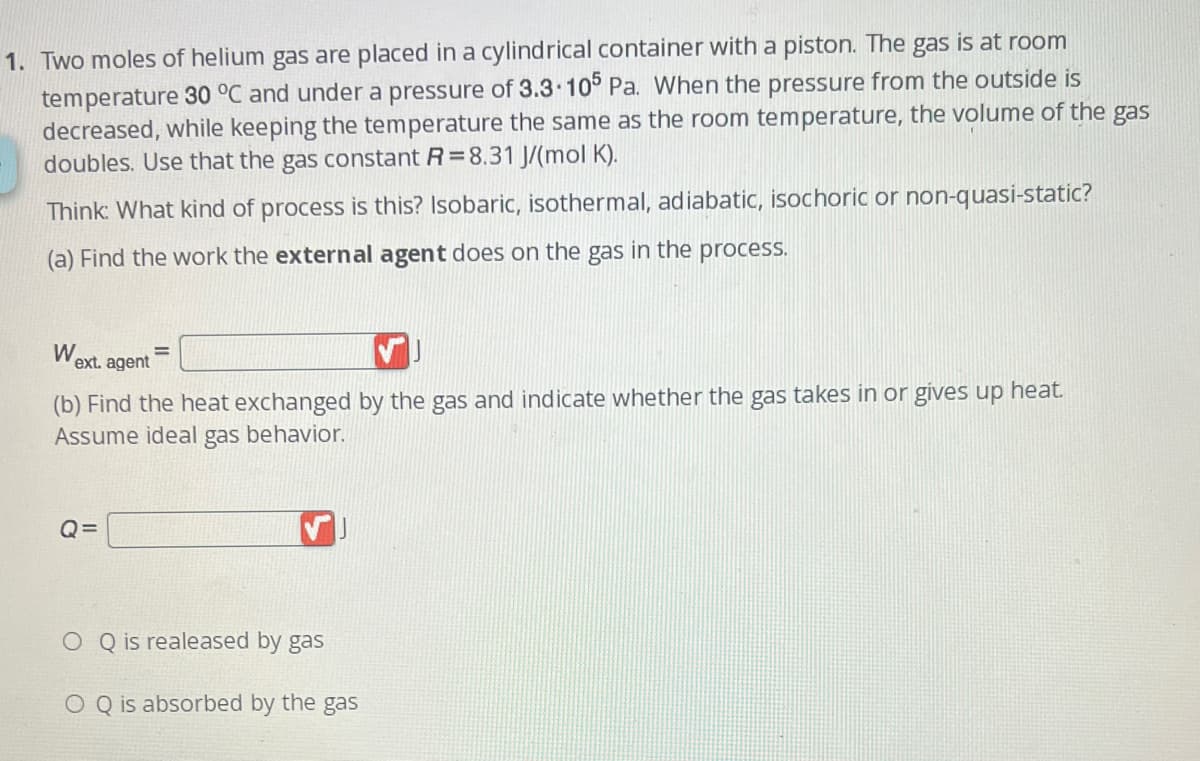
Transcribed Image Text:1. Two moles of helium gas are placed in a cylindrical container with a piston. The gas is at room
temperature 30 °C and under a pressure of 3.3.105 Pa. When the pressure from the outside is
decreased, while keeping the temperature the same as the room temperature, the volume of the gas
doubles. Use that the gas constant R=8.31 J/(mol K).
Think: What kind of process is this? Isobaric, isothermal, adiabatic, isochoric or non-quasi-static?
(a) Find the work the external agent does on the gas in the process.
W
ext. agent
=
(b) Find the heat exchanged by the gas and indicate whether the gas takes in or gives up heat.
Assume ideal gas behavior.
Q=
O Q is realeased by gas
O Q is absorbed by the gas

Transcribed Image Text:1. Two moles of a monatomic ideal gas such as helium is compressed adiabatically and reversibly from
a state (5.5 atm, 4.5 L) to a state with pressure 5.5 atm. For a monoatomic gas y=5/3.
(a) Find the volume of the gas after compression.
V final
(b) Find the work done by the gas in the process.
W=
L.atm
(c) Find the change in internal energy of the gas in the process.
Latm
AE int
Check: What do you predict the signs of work and change in internal energy to be? Do the signs of
work and change in internal energy match with your predictions?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 26 images
