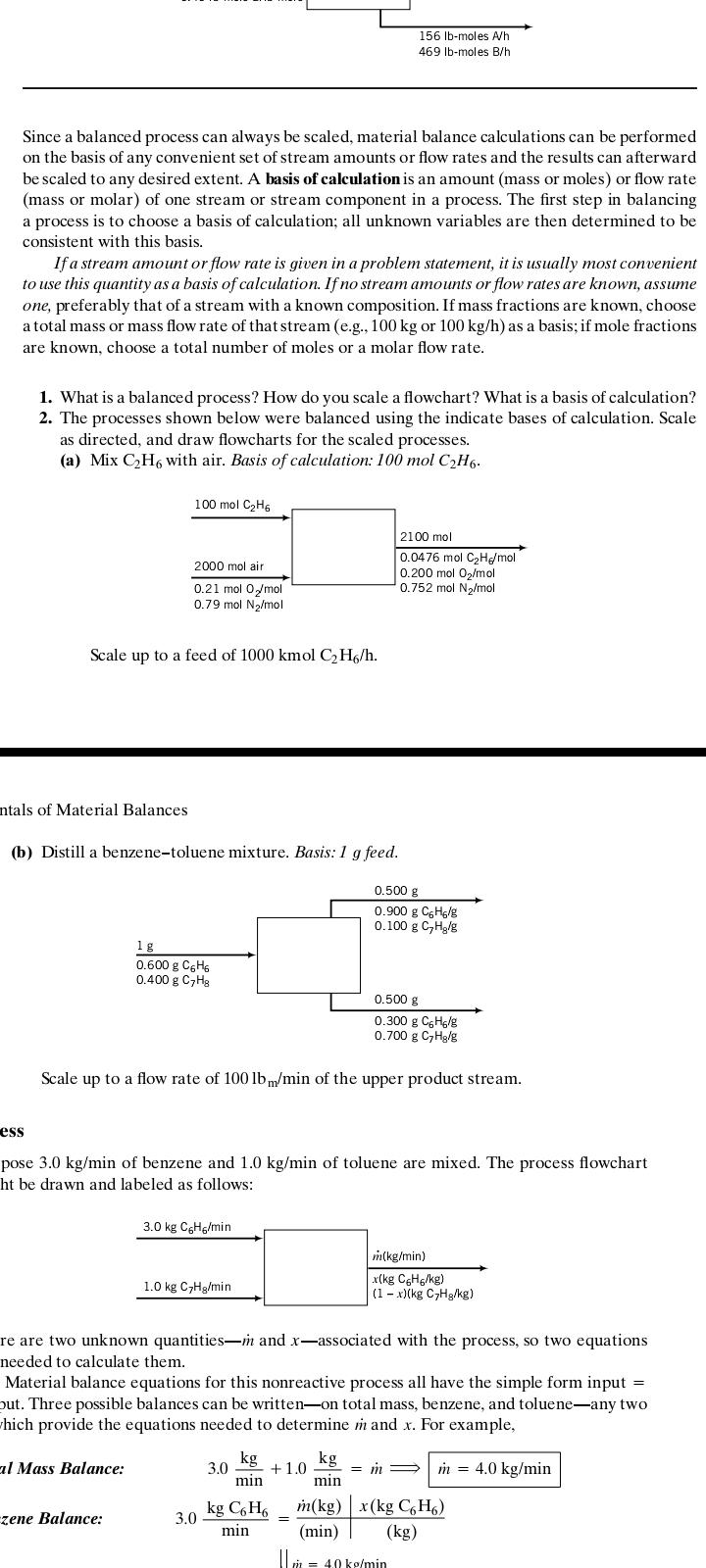
1. What is a balanced process? How do you scale a flowchart? What is a basis of calculation? 2. The processes shown below were balanced using the indicate bases of calculation. Scale as directed, and draw flowcharts for the scaled processes. (a) Mix C₂H6 with air. Basis of calculation: 100 mol C₂H6. 100 mol C₂H6 2000 mol air 0.21 mol O₂/mol 0.79 mol N₂/mol Scale up to a feed of 1000 kmol C₂H6/h. 2100 mol 0.0476 mol C₂He/mol 0.200 mol O₂/mol 0.752 mol N₂/mol
1. What is a balanced process? How do you scale a flowchart? What is a basis of calculation? 2. The processes shown below were balanced using the indicate bases of calculation. Scale as directed, and draw flowcharts for the scaled processes. (a) Mix C₂H6 with air. Basis of calculation: 100 mol C₂H6. 100 mol C₂H6 2000 mol air 0.21 mol O₂/mol 0.79 mol N₂/mol Scale up to a feed of 1000 kmol C₂H6/h. 2100 mol 0.0476 mol C₂He/mol 0.200 mol O₂/mol 0.752 mol N₂/mol
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
Transcribed Image Text:Since a balanced process can always be scaled, material balance calculations can be performed
on the basis of any convenient set of stream amounts or flow rates and the results can afterward
be scaled to any desired extent. A basis of calculation is an amount (mass or moles) or flow rate
(mass or molar) of one stream or stream component in a process. The first step in balancing
a process is to choose a basis of calculation; all unknown variables are then determined to be
consistent with this basis.
If a stream amount or flow rate is given in a problem statement, it is usually most convenient
to use this quantity as a basis of calculation. If no stream amounts or flow rates are known, assume
one, preferably that of a stream with a known composition. If mass fractions are known, choose
a total mass or mass flow rate of that stream (e.g., 100 kg or 100 kg/h) as a basis; if mole fractions
are known, choose a total number of moles or a molar flow rate.
1. What is a balanced process? How do you scale a flowchart? What is a basis of calculation?
2. The processes shown below were balanced using the indicate bases of calculation. Scale
as directed, and draw flowcharts for the scaled processes.
(a) Mix C₂H6 with air. Basis of calculation: 100 mol C₂H6.
ntals of Material Balances
Scale up to a feed of 1000 kmol C₂H6/h.
100 mol C₂H6
2000 mol air
0.21 mol O₂/mol
0.79 mol N₂/mol
(b) Distill a benzene-toluene mixture. Basis: 1 g feed.
1g
0.600 g C₂H₂
0.400 g C₂H₂
al Mass Balance:
zene Balance:
0.500 g
0.300 g C6H6/g
0.700 g C₂Hg/g
Scale up to a flow rate of 100 lbm/min of the upper product stream.
ess
pose 3.0 kg/min of benzene and 1.0 kg/min of toluene are mixed. The process flowchart
ht be drawn and labeled as follows:
3.0 kg C6H6/min
1.0 kg C7Hg/min
re are two unknown quantities-m and x-associated with the process, so two equations
needed to calculate them.
3.0
156 lb-moles A/h
469 lb-moles B/h.
Material balance equations for this nonreactive process all have the simple form input =
put. Three possible balances can be written on total mass, benzene, and toluene-any two
which provide the equations needed to determine m and x. For example,
3.0
kg
min
+1.0
kg C6H6
min
2100 mol
0.0476 mol C₂Hg/mol
0.200 mol O₂/mol
0.752 mol N₂/mol
0.500 g
0.900 g C6H6/g
0.100 g C₂H₂/g
=
kg
min
m(kg/min)
x(kg C6H6/kg)
(1-x)(kg C7Hg/kg)
= m => in = 4.0 kg/min
11m m 40 kg/min
=
m(kg) x(kg C6H6)
(min)
(kg)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The