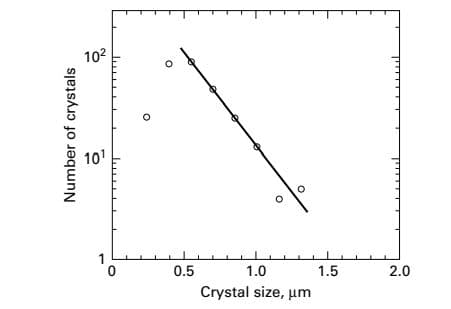
102 101 0.5 1.0 1.5 2.0 Crystal size, um Number of crystals
Tsuge and Matsuo studied precipitation of Mg(OH)2 by reacting aqueous solutions of MgCl2 and Ca(OH)2 in a 1-L MSMPR crystallizer operating at 450 rpm and 25oC. Crystal sizes were measured by a scanning-electron microscope (SEM) and analyzed by a digitizer. Crystal size was taken to be the maximum length. A plot of the crystal-size distribution is given in Figure for an assumed residence time of 5 minutes. Assuming that the number of crystals is proportional to exp(-L/Gτ), as in n=noexp(-L/Gτ), determine: (a) growth rate, (b) nucleation rate, and (c) predominant crystal size.
Magnesium hydroxide is precipitated by reacting of aqueous solution of MgCl2 and Ca(OH)2 in a 1-L MSMPR crystallizer.
The operating conditions of crystallizer are given as:
Temperature = 25 0C
Impeller speed = 450 rpm
The crystal size distribution is given in the figure.
Residence time = 5 min
Number of crystal is equal to,
n = n0e(-L/Gτ)
(a)
Growth rate of the crystal is calculated as follows:
The equation to calculate the number of crystal is given as,
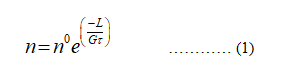
Where,
n represents the number of crystal.
n0 represents the proportionality constant.
L represents the length.
G represents the growth rate.
Ττ represents the residence time
The proportionality constant is also equal to,
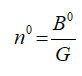
Where,
B0 represents the nucleation rate.
The equation (1) becomes,
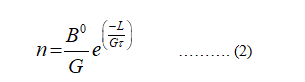
Take the log on the both sides of the equation (2)
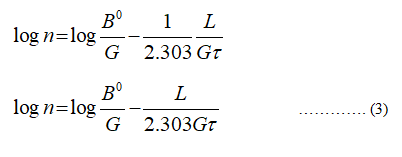
Equation (3) represents a straight equation (y = mx +c)
Where,
y = n
m= G/2.303τ
x = l
c = log B0/G
The values of unknowns are calculated as follows:
Take two values (extreme points) of number of crystals and corresponding values of the crystal size from the graph given in the question.
For n = 90
L = 0.6
For n = 3
L = 1.3
The residence time = 5 min
Plugin the values in equation (3)
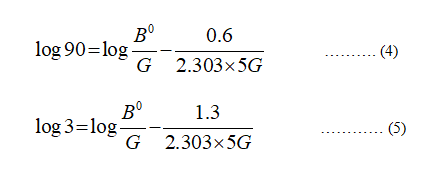
Step by step
Solved in 6 steps with 9 images









