198 The half-life of Au is 2-7 days. Calculate (a) the decay constant, (b) the average-life and (c) the activity of 1-00 mg of 198 198 Au. Take atomic weight of Au to be 198 g mol.
Q: Calculate the angular speed of minute hand clock. If minute hand is 5 cm long, calculate linear velo...
A: Here we have to find, 1) Linear velocity tip of minute hand (v) 2) Angul...
Q: The spacing between atomic planes in a crystal is 0.130 nm. a) If 15.0 keV x rays are diffracted by...
A: Solution: From Bragg's law of diffraction, the spacing between the atomic planes is related to the w...
Q: A copper wire of 50m length with a cross-sectional area of 8.17 x 10-7m2, carries a current of 1.67A...
A: In this case, a copper wire of some length and a certain cross-sectional area carries some current. ...
Q: The energy levels of hydrogen atom ( including fine structure term ) takes the following form 13.6ev...
A: Answer: It is given the energy for hydrogen atom including spectral term. We have to find the energ...
Q: 16.x e, let D = 8xyz"ax+4.x"z*ay + 16x hrough the rectangular surface z = E at P(2,-1, 3). (c) Find ...
A: Given: The equation in the free space is 8xyz4ax+4x2z4ay+16x2yz3 pC/m2
Q: 2. A block of mass m rests on a plane inclined at 0 with the horizontal. The block is attached to a ...
A: Solution:-Given that Free Body diagram of block:- (FBD-1)
Q: A volume of 58.0 L of hydrogen is heated from 33°C to 68°C. If its original density is 4.85 kg/m3 an...
A: The following data are given: Initial Volume, V1=58L=0.058m3 Initial Temperature, T1=33°C=306K Final...
Q: 5. A circular conducting coil, with the radius R 15.0 cm, is placed in a uniform veriti magnetic fie...
A: Here the magnetic field is acting into the plane of the paper, The magnitude of the magnetic field i...
Q: Calculate the approximate temperatures at which the following energy storage modes of a nitrogen mol...
A: We know that,thermal energy corresponding to the energyE=12kTk is Boltzman constantk=8.617×10-5eV/Kh...
Q: (b) The ground state of the fluorine atom is represented by the level 2 P3/2. It is subject to a wea...
A: Note :- Since we only answer up to 3 sub-parts, we’ll answer the first 3. Please resubmit the questi...
Q: . The radiative transfer equation is written as, dl, ds -a,l, + ju. Show that this equation 1 can be...
A:
Q: For the given arrangement, find out the path travelled by the incident ray within the slab in meter:...
A: Refer to the figure below : Applying Snell's law at the interface of air and first medium : sinθs...
Q: The equipartition theorem of energy in classical statistical mechanics says that the contribution to...
A: Given, Mixture of gases, is ideal, classical and diatomic. Molecules=Nα Where α=1,2,..., c Specific ...
Q: Monochromatic light is incident on two identical and parallel slits of Young's double-slit setup. Th...
A: Monochromatic light incident on the parallel slit setup is diffracted by the two slits. The diffract...
Q: A car making a turn on a dry, banked highly ramp is experiencing friction. The coefficient of static...
A: radius of the road, r=195 m The coefficient of friction between the tires and the road, μ=0.58 The b...
Q: II II IV VI 2. Five infinite parallel planes of charge have surface charge densities o, -o, o, -o, o...
A: Electric field due to plane of charge, E=σ2ε0 The electric field is away from the plate for the posi...
Q: A positive charge + q is located at the point x = 0, y = -a, and a negative charge -q is at the poin...
A: Refer to the figure below :
Q: heavy pulley puck Dot number Displacement of the puck Time Angular displacement of the pulley t (sec...
A: The data values of the sixth data point are given as : s=0.14m, t=0.6s, θ=4.5751rad The angular ...
Q: digital spectrometer have the peak value for one of the wavelengths is found to be 489 nm. The width...
A: Given that, The minimum wavelength is given by 3.0 nm. The maximum wavelength is given by 1.5 nm. Th...
Q: You are observing a different star which has a normal measured flux of 29.7 photons per second. The ...
A: Solution: From Kepler's third law, the orbital radius and the period are related as the following: P...
Q: 61 : A metal wire of length 2.5 m and area of cross section 1.5 × 10 m² is stretched through 2 mm. C...
A: Work done in stretching the wire gets stored in the form of its elastic potential energy. It is some...
Q: A model train engine was moving at a constant speed on a straight horizontal track. As the engine mo...
A: The formula for the time of flight of the projectile is given by T=2usinθg where u is the initial ve...
Q: 2.- Two metallic spheres with mass m1= 1kg and m2= 2kg are fixed at the ends of a mass-less rod at d...
A: Given that,mass, m1=1 kgmass, m2=2 kglength, l=3 cmlet consider origin at m1then, lcm=m1×0+m2×3m1+m2...
Q: Draw a circuit diagram with 2 cells in series, 3 light bulb in parallel, 3 ammeters placed so that t...
A: An ammeter measures current when placed in series with the element of the circuit across which curre...
Q: A tube open at both ends has length 47 cm. calculate the fundamental frequency of air column. (Negl...
A: The length of the air column is equal to one-fourth of the wavelength for the first harmonic. The fu...
Q: (Q) Consider a system of three noninteracting particles that are confined to move in a one-dimension...
A:
Q: Find the total current in the circuit below. 60 50 70 40 30 40 30. 100S 100 120 30 50 70 20 120 V 70...
A:
Q: A uniform magnetic field of magnitude 0.14 T is directed along the positive x-axis. A positron movin...
A: Given that, Magnetic field, B=0.14TSpeed, v=4.95×106 m/sAngle, θ=85°Pitch, p=2πmvCosθqB Equat...
Q: Consider the following cireuit: DDDD G. a. Write the truth table for G(X.YZ). b. Convert the circuit...
A: Given, Logic gate is, So, truth table for output G(X,Y,Z), Input Output X Y Z G 0 0 0 0 ...
Q: A spherical refracting surface separating air from index 1.523 has a radius of curvature of+100 mm. ...
A: The sagitta, or sag, of a convex or concave lens surface refers to the height (or depth) of such sur...
Q: liquid, the colum.
A: To give the reason when a cold alcohol thermometer is placed in a hot liquid, the column of alcohol ...
Q: Solar input power to the PV Module is 1200W, this PV Module is producing an output power of 228W the...
A: Photovoltaic solar cells convert sunlight into electricity. Ther are made of photosensitive material...
Q: A mass of 5kg is moving in a circle of radius 1m. If the centripetal force acting on the mass is 20N...
A: Given that, Force F = 20 N, Radius r = 1 m, Mass ...
Q: A cube, whose mass is 0.580 kg, is attached to a spring with a force constant of 104 N/m. The cube r...
A: Solution: a). If there is elongation or compression (x) in the spring, the restoring force acted by ...
Q: 6 : The elastic limit of copper is 1.5 x 10' N/m². Find the maximum radius of the copper wire must h...
A: Given Data : F=10 kg ωt=10×9...
Q: To stimulate the acceleration of large rockets, astronauts are spun at thr end of a long rotating be...
A: Given that, r=10 mCentripetal acceleration=8×g We have to find angular speed (ω)
Q: RT Constant Temperature. If the temperature throughout the gas remains constant (isothermal) at T=T ...
A: Solution: The pressure P is given as the following: P=P0e-gRT0z-z0put, g=g0r2r+z2 P=P0e-g0r2r+z2RT0z...
Q: E(r) = Bzî – ax²y'j+ (6ayxy + Bóz") k %3D where a, B, y and & are constants and r = xi+yj+zk. Find t...
A: Solution:-Given thatE(r)=βzi^-αx2y3j^+(6αγxy+βδz3)k^α, β, γ and δ are constants.r=xi^+yj^+zk^
Q: You hold an elliptical wire loop in the same two-dimensional x-y plane as a very long wire of consta...
A:
Q: A 30-cm long solenoid has 600 turns. If you put 4 A of current through the wires, what would be the ...
A: Given that,Length : L = 30 (cm)Here,As we know that, n = NLPutting the valu...
Q: In which case does the electric field reach very high values? at sharp points A) B) at convex points...
A: Electric field is the area or field surround a electric charged particle that exert some force on th...
Q: Dot number Displacement of the puck Time Angular displacement of the pulley s (m) 1 (sec) e (rad) 3 ...
A:
Q: Given a sin-wave that has its maximum at x, = 2 cm at t = 0 %3D sec. Calculate the initial phase ang...
A: Given, wavelength, λ = 18cm=0.18m At, xo = 0.02(m) and t=0, amplitude is maximum, And this sinusoida...
Q: A block with a mass of 44.5 kg is pushed with a horizontal force of 150 N. The block moves at a cons...
A: The block is being pushed across the floor, by the application of a horizontal force. This horizonta...
Q: A pitcher throws a 0.200 kg ball so that its speed is 13.0 m/s and angle is 40.0° below the horizont...
A: Given that, Mass of the ball, m=0.2 kg Initial speed, u=13 m/s Angle, θ=40°. Final speed, ν=50 m/sA...
Q: A piece of wood is 0.570 m long, 0.250 m wide, and 0.076 m thick. Its density is 700 kg/m3. What vol...
A: Given that:Length, L=0.570 mWidth, w=0.250 mThickness, t=0.076 mDensity of wood, ρB=700 kg/m3Density...
Q: A graduate student in geology who grew up in Florida (in the southern most tip of the United States)...
A: We know that, At the north pole, the celestial equator lies on the horizon. Slowly on latitude obser...
Q: Match each of the following wave functions (41, 42, 43, and 44) with the appropriate bond (A, B, C, ...
A: We will be using the coordinates of the end points of the bonds to determine the wavefunctions. We w...
Q: The delivery of Tc-99m (6 hour half life) unit doses is delayed by a snowstorm for 30 minutes. Addit...
A: Half life of the unit dose=6 hour T1/2=6 hours=6×60×60=21600 sThe decay constant, λ=ln 2T1/2λ=0.6932...
Q: e Canadians troups are sent (as part of a U.N. peacekeeping force) to a country located on the Earth...
A: The shape of the Earth is spherical in shape but is flattened at the poles. The rotation of the eart...
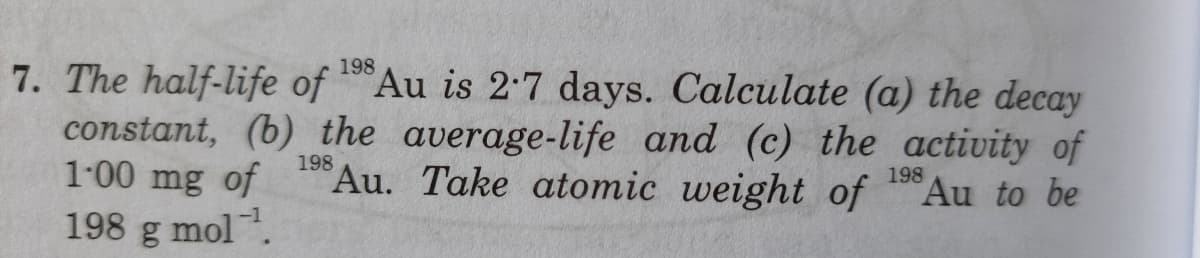
Step by step
Solved in 4 steps
