2 Hydrogen Atom Eigenstates The state of an electron in a hydrogen atom at time t = 0 is given by |6(0)) = (12.,0,0) + C 12, 1,-1) – (3, 1,0)), where the states In,1,m) are the simultaneous energy, angular momentum and component of angular momentum eigenstates for the electron in the hydrogen atom. (a) Find the real and positive C that normalizes the state. (b) What are (E), (L²), and (L.) for this state? (c) Which of these change as a function of time?
2 Hydrogen Atom Eigenstates The state of an electron in a hydrogen atom at time t = 0 is given by |6(0)) = (12.,0,0) + C 12, 1,-1) – (3, 1,0)), where the states In,1,m) are the simultaneous energy, angular momentum and component of angular momentum eigenstates for the electron in the hydrogen atom. (a) Find the real and positive C that normalizes the state. (b) What are (E), (L²), and (L.) for this state? (c) Which of these change as a function of time?
Related questions
Question
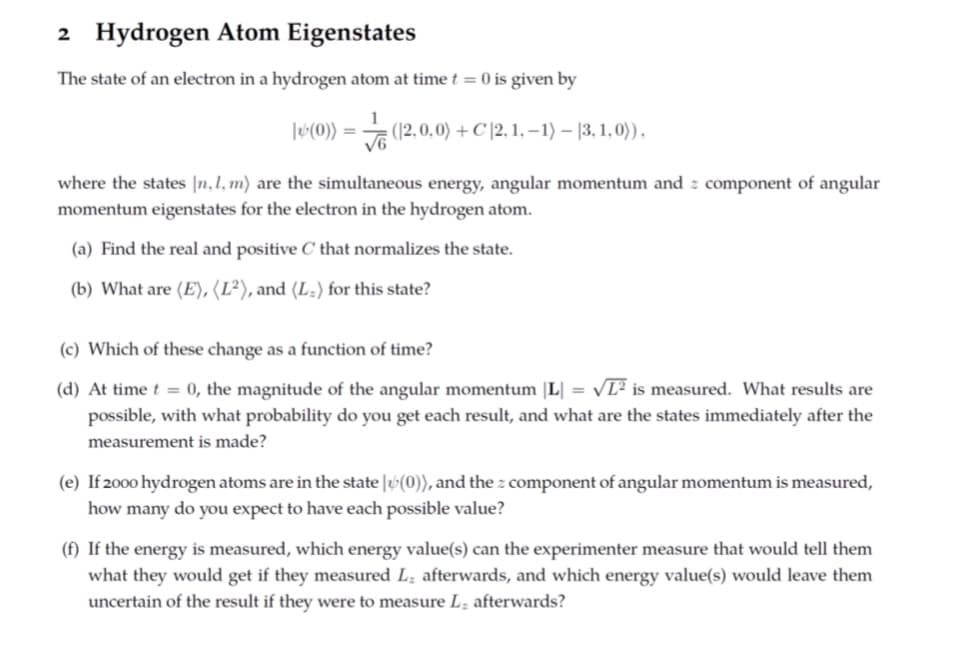
Transcribed Image Text:2 Hydrogen Atom Eigenstates
The state of an electron in a hydrogen atom at time t = 0 is given by
|*(0)) = √ (12,0,0) + C |2, 1, −1) — (3, 1,0)),
where the states [n, 1, m) are the simultaneous energy, angular momentum and component of angular
momentum eigenstates for the electron in the hydrogen atom.
(a) Find the real and positive C that normalizes the state.
(b) What are (E), (L²), and (L-) for this state?
(c) Which of these change as a function of time?
=
(d) At time t = 0, the magnitude of the angular momentum |L| VL² is measured. What results are
possible, with what probability do you get each result, and what are the states immediately after the
measurement is made?
(e) If 2000 hydrogen atoms are in the state (0)), and the component of angular momentum is measured,
how many do you expect to have each possible value?
(f) If the energy is measured, which energy value(s) can the experimenter measure that would tell them
what they would get if they measured L. afterwards, and which energy value(s) would leave them
uncertain of the result if they were to measure L, afterwards?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images
