2.14 Consider the piston-cylinder arrangement shown in the figure. A frictionless piston is free to move between two sets of stops. When the piston rests on the lower stops, the enclosed volume is 400 L. When the piston reaches the upper stops, the volume is 600 L. The cylinder initially contains water at 100 kPa, 20% quality. It is heated until the water eventually exists as saturated vapor. The mass of the piston requires 300 kPa pressure to move it against the outside ambient pressure. Determine the final pressure in the cylinder, the heat transfer (in kJ), and the work (in kJ) for the overall process.
2.14 Consider the piston-cylinder arrangement shown in the figure. A frictionless piston is free to move between two sets of stops. When the piston rests on the lower stops, the enclosed volume is 400 L. When the piston reaches the upper stops, the volume is 600 L. The cylinder initially contains water at 100 kPa, 20% quality. It is heated until the water eventually exists as saturated vapor. The mass of the piston requires 300 kPa pressure to move it against the outside ambient pressure. Determine the final pressure in the cylinder, the heat transfer (in kJ), and the work (in kJ) for the overall process.
Refrigeration and Air Conditioning Technology (MindTap Course List)
8th Edition
ISBN:9781305578296
Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Chapter2: Matter And Energy
Section: Chapter Questions
Problem 30RQ: A gas is compressed inside a compressor's cylinder. When the piston is at its bottom dead center,...
Related questions
Question
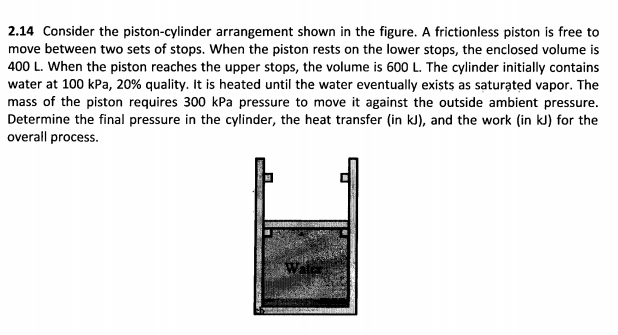
Transcribed Image Text:2.14 Consider the piston-cylinder arrangement shown in the figure. A frictionless piston is free to
move between two sets of stops. When the piston rests on the lower stops, the enclosed volume is
400 L. When the piston reaches the upper stops, the volume is 600 L. The cylinder initially contains
water at 100 kPa, 20% quality. It is heated until the water eventually exists as saturated vapor. The
mass of the piston requires 300 kPa pressure to move it against the outside ambient pressure.
Determine the final pressure in the cylinder, the heat transfer (in kJ), and the work (in kJ) for the
overall process.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Refrigeration and Air Conditioning Technology (Mi…
Mechanical Engineering
ISBN:
9781305578296
Author:
John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:
Cengage Learning

Refrigeration and Air Conditioning Technology (Mi…
Mechanical Engineering
ISBN:
9781305578296
Author:
John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:
Cengage Learning