3. Find the dew and bubble temperatures of a mixture 85% mol water and 15% mol acetic acid at Ibar. There is a non-ideal behavior in the liquid phase. The activity coefficients of water and acetic acid are given by: logy = x² B+C(4Xw − 1) + C(Xw − X₂ )(6x₁ − 1) logy₁ = x² B+C(4.xw − 3) + C(xw − x₁)(6.x - 5) where the W and A subscripts are for water and acetic acid, x is the mol fraction and: 43.27 B=0.1735- T(K) C = 0.1081 Use the Antoine equation to get vapor pressures of water and acetic acid. Constants can be found below
3. Find the dew and bubble temperatures of a mixture 85% mol water and 15% mol acetic acid at Ibar. There is a non-ideal behavior in the liquid phase. The activity coefficients of water and acetic acid are given by: logy = x² B+C(4Xw − 1) + C(Xw − X₂ )(6x₁ − 1) logy₁ = x² B+C(4.xw − 3) + C(xw − x₁)(6.x - 5) where the W and A subscripts are for water and acetic acid, x is the mol fraction and: 43.27 B=0.1735- T(K) C = 0.1081 Use the Antoine equation to get vapor pressures of water and acetic acid. Constants can be found below
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
63
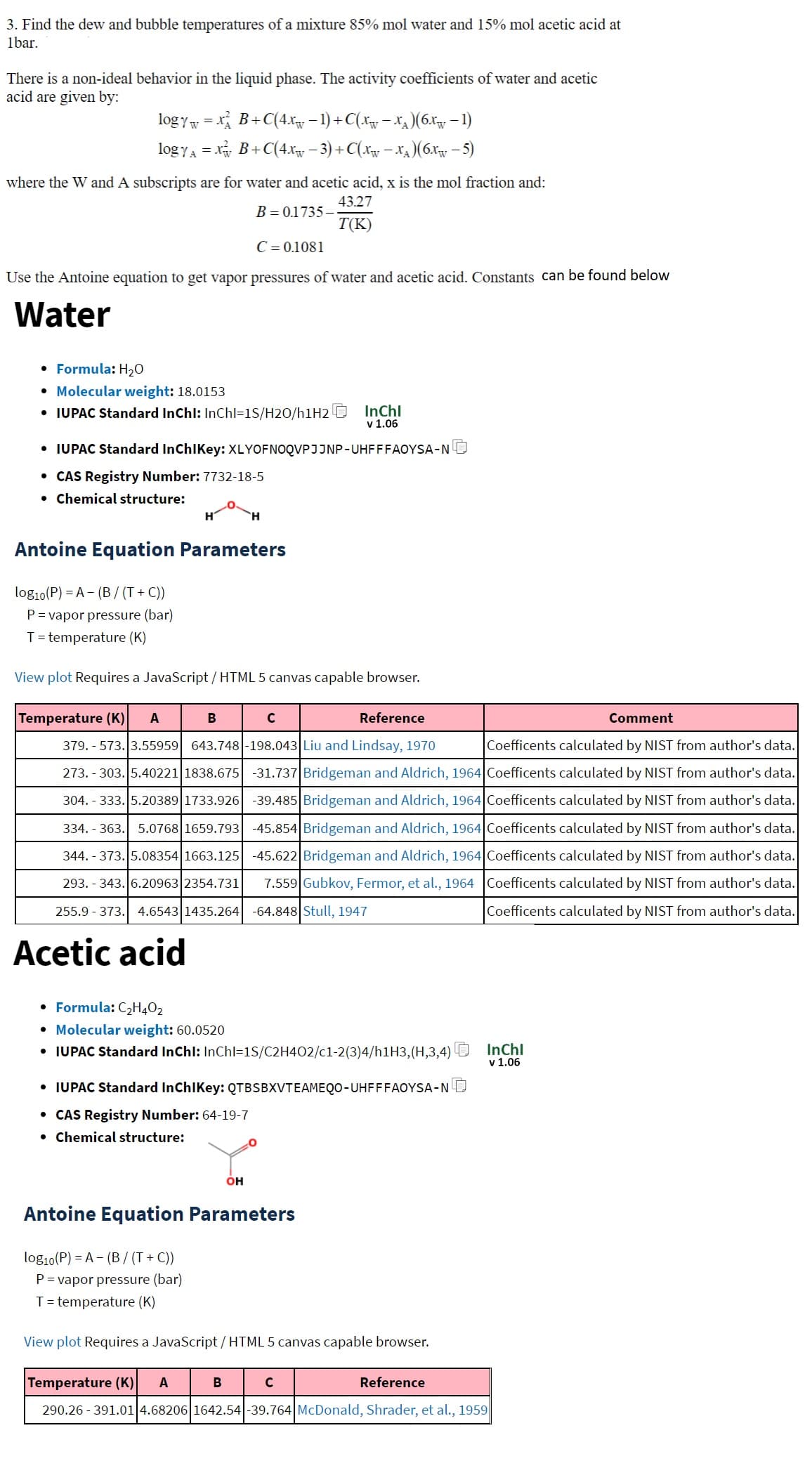
Transcribed Image Text:3. Find the dew and bubble temperatures of a mixture 85% mol water and 15% mol acetic acid at
Ibar.
There is a non-ideal behavior in the liquid phase. The activity coefficients of water and acetic
acid are given by:
logy = x B+C(4x − 1) + C(x − x)(6x − 1)
logy₁ = x² B+C(4xw − 3) + C(xw − x₁)(6x – 5)
A
where the W and A subscripts are for water and acetic acid, x is the mol fraction and:
43.27
B = 0.1735-
T(K)
C = 0.1081
Use the Antoine equation to get vapor pressures of water and acetic acid. Constants can be found below
Water
• Formula: H₂O
• Molecular weight: 18.0153
• IUPAC Standard InChl: InChl=1S/H20/h1H2
• IUPAC Standard InChIKey: XLYOFNOQVPJJNP-UHFFFAOYSA-N
• CAS Registry Number: 7732-18-5
● Chemical structure:
log₁0 (P) = A (B/ (T+C))
P = vapor pressure (bar)
T = temperature (K)
H
Antoine Equation Parameters
View plot Requires a JavaScript/HTML 5 canvas capable browser.
H
B
Temperature (K) A
Reference
Comment
379.-573. 3.55959 643.748-198.043 Liu and Lindsay, 1970 Coefficents calculated by NIST from author's data.
273.303. 5.40221 1838.675 -31.737 Bridgeman and Aldrich, 1964 Coefficents calculated by NIST from author's data.
304. - 333.5.20389 1733.926 -39.485 Bridgeman and Aldrich, 1964 Coefficents calculated by NIST from author's data.
334.-363. 5.0768 1659.793 -45.854 Bridgeman and Aldrich, 1964 Coefficents calculated by NIST from author's data.
344.-373. 5.08354 1663.125 -45.622 Bridgeman and Aldrich, 1964 Coefficents calculated by NIST from author's data.
293. 343. 6.20963 2354.731 7.559 Gubkov, Fermor, et al., 1964 Coefficents calculated by NIST from author's data.
255.9373. 4.6543 1435.264 -64.848 Stull, 1947
Coefficents calculated by NIST from author's data.
Acetic acid
log10(P) = A (B/(T+C))
P = vapor pressure (bar)
T = temperature (K)
• Formula: C₂H4O₂
• Molecular weight: 60.0520
• IUPAC Standard InChl: InChl=1S/C2H4O2/c1-2(3)4/h1H3, (H,3,4)
InChl
v 1.06
C
• IUPAC Standard InChIKey: QTBSBXVTEAMEQO-UHFFFAOYSA-N
• CAS Registry Number: 64-19-7
● Chemical structure:
OH
Antoine Equation Parameters
B
с
View plot Requires a JavaScript/HTML 5 canvas capable browser.
Reference
Temperature (K) A
290.26-391.01 4.68206 1642.54-39.764 McDonald, Shrader, et al., 1959
InChl
v 1.06
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The