4) Molybdenum has a BCC crystal structures, the density of molybdenum is 10.22 g/cm³ and its atomic mass is 95.94 g/mol. What are the atomic concentration, the lattice parameter a and the atomic radius of molybdenum. What is the atomic concentration in the primitive cell? 5) Tungsten (W) has the BCC crystal structure. The radius of the W atom is 0.1371 nm. The atomic mass of W is 183.8 amu (g/mol). Calculate the number of W atoms per unit volume and the density of W (NA = 6.02 x 10²3).
4) Molybdenum has a BCC crystal structures, the density of molybdenum is 10.22 g/cm³ and its atomic mass is 95.94 g/mol. What are the atomic concentration, the lattice parameter a and the atomic radius of molybdenum. What is the atomic concentration in the primitive cell? 5) Tungsten (W) has the BCC crystal structure. The radius of the W atom is 0.1371 nm. The atomic mass of W is 183.8 amu (g/mol). Calculate the number of W atoms per unit volume and the density of W (NA = 6.02 x 10²3).
Related questions
Question
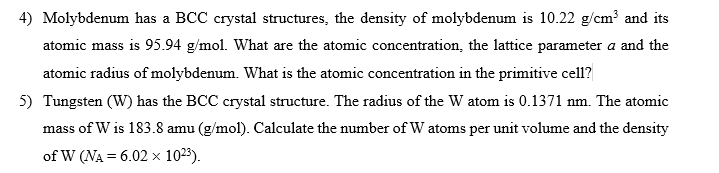
Transcribed Image Text:4) Molybdenum has a BCC crystal structures, the density of molybdenum is 10.22 g/cm³ and its
atomic mass is 95.94 g/mol. What are the atomic concentration, the lattice parameter a and the
atomic radius of molybdenum. What is the atomic concentration in the primitive cell?
5) Tungsten (W) has the BCC crystal structure. The radius of the W atom is 0.1371 nm. The atomic
mass of W is 183.8 amu (g/mol). Calculate the number of W atoms per unit volume and the density
of W (NA = 6.02 x 10²3).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images
