5. Write a balanced equation for each of the processes in Part A. Remember to include heat on the reactant or product side, as appropriate.
5. Write a balanced equation for each of the processes in Part A. Remember to include heat on the reactant or product side, as appropriate.
Anatomy & Physiology
1st Edition
ISBN:9781938168130
Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark Womble
Publisher:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark Womble
Chapter24: Metabolism And Nutrition
Section: Chapter Questions
Problem 24RQ: The heat you feel on your chair when you stand up was transferred from your skin via ________....
Related questions
Question
Answer number 5

Transcribed Image Text:Exploring Energy Changes-Page 6
Post-Lab Questions
Attach the printout of the data table and graph for Part B to your lab report.
1. Complete the following Results Table to indicate whether each reaction in Part A repre-
sents a physical or chemical change and whether it is exothermic or endothermic.
Reaction
Physical or Chemical Change?
Exothermic or Endothermic?
Physical Charge
Physical ihangE
Chemical.change
Endothermi
Exothermic
A
C
Endathermicc
2. A chemical change involves a change in the composition of matter-the formation of a
new chemical substance (product) with physical and chemical properties different from
those of the reactants. Describe the evidence used to decide if any of the processes in Part
A were chemical changes.
3. Did you observe any qualitative differences in the amount of heat generated in the reac-
tions that were characterized as endothermic in Part A?
4. Consider Reaction A: Was energy released or absorbed by the reactants in this system?
When you touched the reaction container (the plastic bag) was energy being released or
absorbed by your hand?
5. Write a balanced equation for each of the processes in Part A. Remember to include heat
on the reactant or product side, as appropriate.
6. In Part B, was the temperature that was measured a part of the
Transcribed Image Text:Exploring Energy Changes
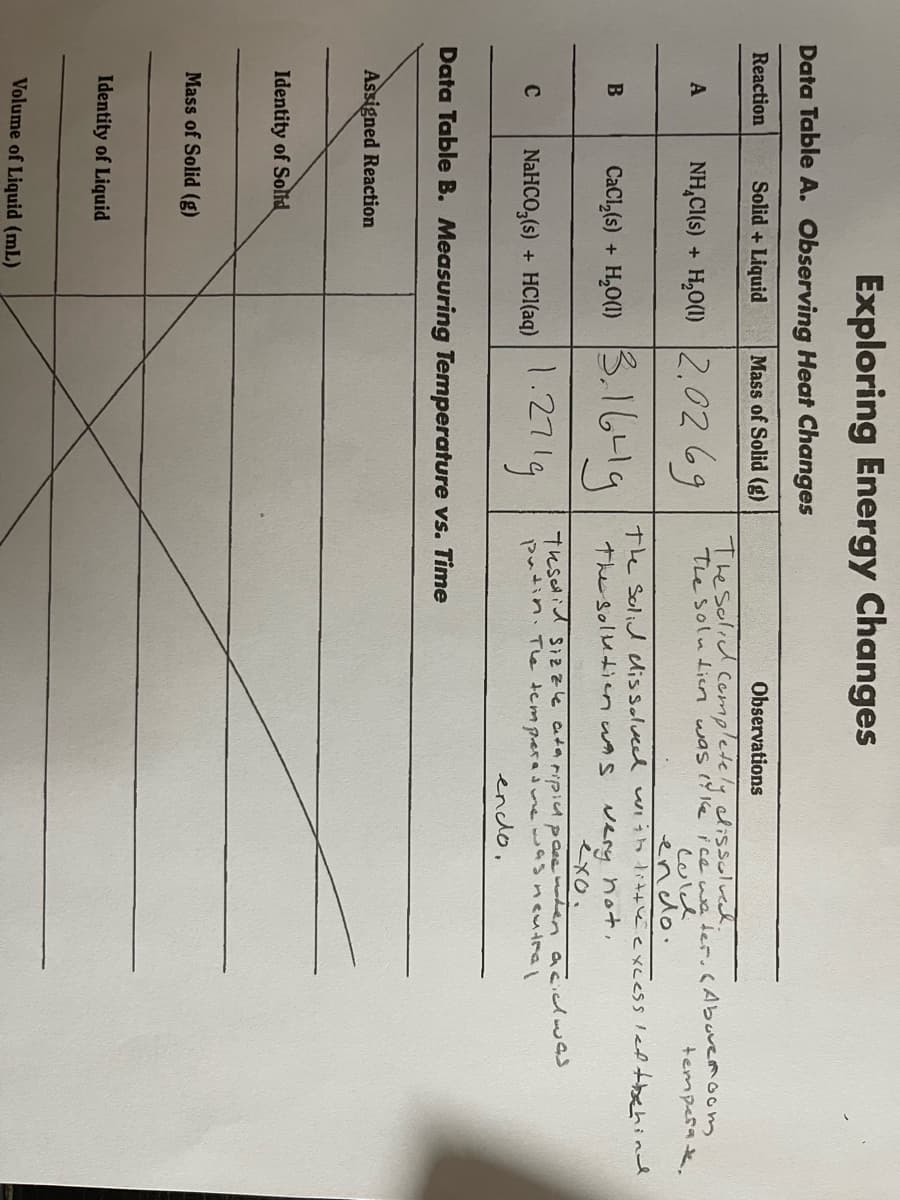
Data Table A. Observing Heat Changes
Reaction
Solid + Liquid
Mass of Solid (g)
Observations
Thesolid Cempletely dissolved.
The soluien was ( 1e ice we ter.CAbuvemoom
Culd
endo.
The Solid lissolveed uiih little excess led tbehind
NH,CI(s) + H,O(1) 2.0269
A
temperak.
CacıĻ6) + 6-l9
H,O(1) 3.1
3.1641g
very hot,
exo.
The solutien wAs
NaHCO,(6) + HCl(aq) 271g
Tesid Sizzle ataripicA pase er acidwas
putin. Tle tempera mewasncutral
enclo,
C
NaHCO,(s) + HCI(aq)
Data Table B. Measuring Temperature vs. Time
Assigned Reaction
Identity of Solid
Mass of Solid (g)
Identity of Liquid
Volume of Liquid (mL)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Anatomy & Physiology
Biology
ISBN:
9781938168130
Author:
Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark Womble
Publisher:
OpenStax College

Biology: The Unity and Diversity of Life (MindTap…
Biology
ISBN:
9781337408332
Author:
Cecie Starr, Ralph Taggart, Christine Evers, Lisa Starr
Publisher:
Cengage Learning

Biology: The Unity and Diversity of Life (MindTap…
Biology
ISBN:
9781305073951
Author:
Cecie Starr, Ralph Taggart, Christine Evers, Lisa Starr
Publisher:
Cengage Learning

Anatomy & Physiology
Biology
ISBN:
9781938168130
Author:
Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark Womble
Publisher:
OpenStax College

Biology: The Unity and Diversity of Life (MindTap…
Biology
ISBN:
9781337408332
Author:
Cecie Starr, Ralph Taggart, Christine Evers, Lisa Starr
Publisher:
Cengage Learning

Biology: The Unity and Diversity of Life (MindTap…
Biology
ISBN:
9781305073951
Author:
Cecie Starr, Ralph Taggart, Christine Evers, Lisa Starr
Publisher:
Cengage Learning